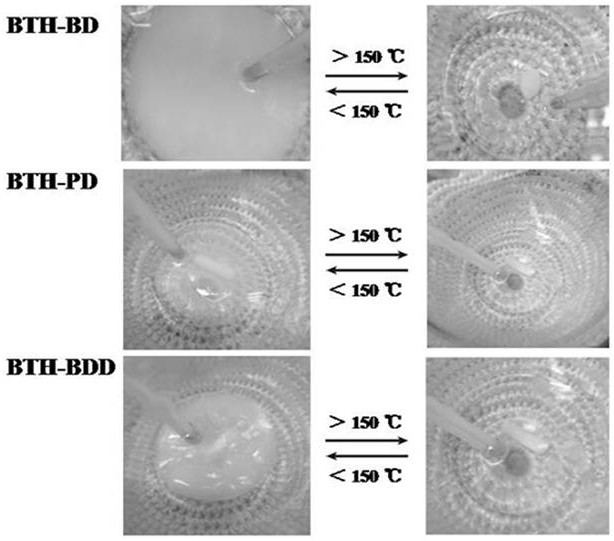
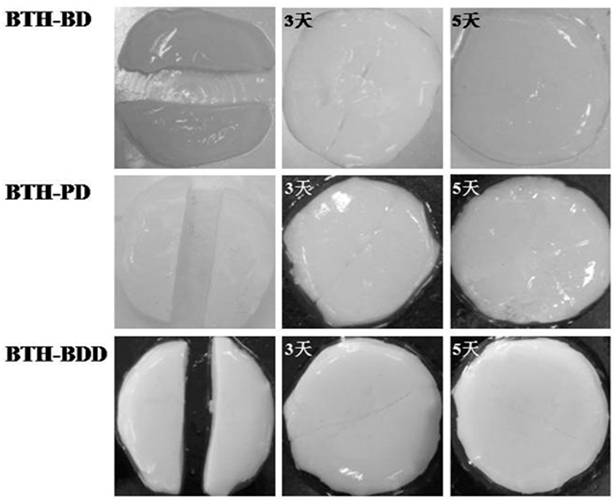
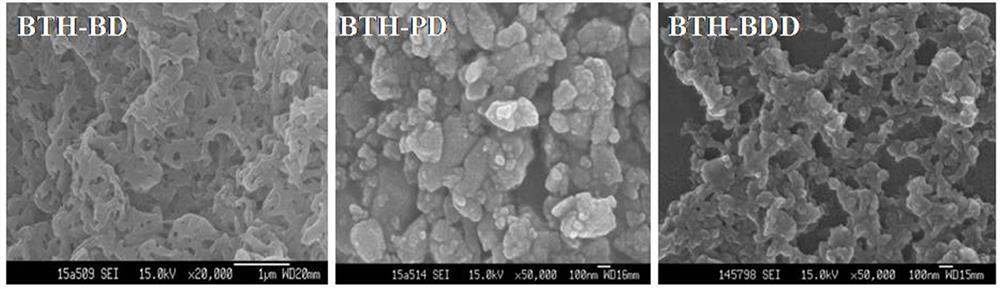
Preparation method of novel acylhydrazone bond gel through one-step cross-linking polymerization
A technology of cross-linking polymerization and acylhydrazone bond is applied in the field of preparation of new cross-linking polymerization acylhydrazone bond gel to achieve high yield, good physical and chemical properties, cutting-edge design ideas and novel effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of triethyl trimesate: Weigh 4.53 g (17.0 mmol) of 1,3,5-benzenetricarboxylic acid chloride in a 150 mL round bottom flask, add 50 mL of absolute ethanol and 5-8 drops of triethylamine, The reaction was stirred at room temperature for 6 h, and the reaction was stopped to obtain a white solid, which was filtered with suction, and then recrystallized with absolute ethanol and filtered to obtain white needle-shaped crystals; the synthetic route is as follows.
[0035]
Embodiment 2
[0037] Synthesis of trimesohydrazide: Weigh 1.47 g (5.0 mmol) of triethyl trimesate into a 150 mL round bottom flask, add 20 mL of 80% hydrazine hydrate, 30 mL of absolute ethanol and 30 mL of tetrahydrofuran, stir Heating under reflux for 6 h, distilling off ethanol under reduced pressure, cooling to precipitate a large amount of white solid, suction filtration, and then washing with a large amount of water until neutral, then recrystallizing the solid with absolute ethanol and filtering to obtain a white solid powder; its synthesis The route is shown below.
[0038]
Embodiment 3
[0040] Preparation of a new type of acylhydrazone bonded polymer gel (BTH-BD): Weigh 100.5 mg (0.4 mmol) of trimellitic hydrazide into a 10 mL glass vial, add 4.0 mL of DMSO solvent, and dissolve completely by ultrasonication for 10 min; then weigh Put 80.8 mg (0.6 mmol) of isophthalaldehyde in another 10 mL glass vial, add 4.0 mL DMSO solvent, and dissolve completely. Under ultrasound, the DMSO solution of isophthalaldehyde was slowly added dropwise to the DMSO solution of trimellitic hydrazide, and the mixed solution gradually changed from a cloudy solution to a light green transparent solution. gel state, and then transfer the newly prepared gel to an oven for heating and aging at 80 ºC for 12 h. After aging, take out the solvent in the vial, soak and wash the gel with 15-30 mL tetrahydrofuran for 24 h (3 times, exchange the solvent every 8 h); then soak and wash the gel with 15-30 mL absolute ethanol for 24 h ( 3 times, the solvent was exchanged every 8 h). Finally, free...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com