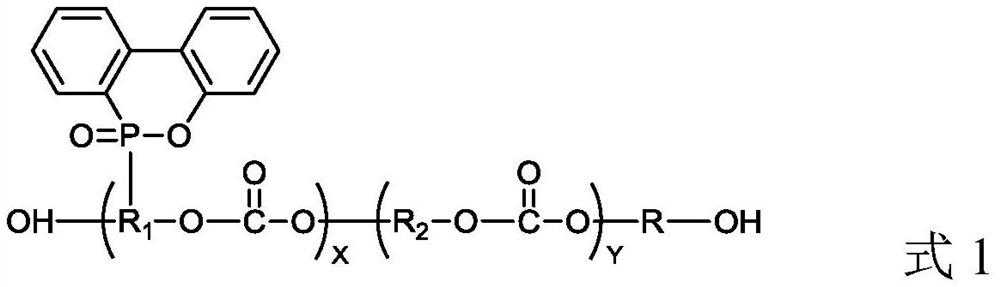
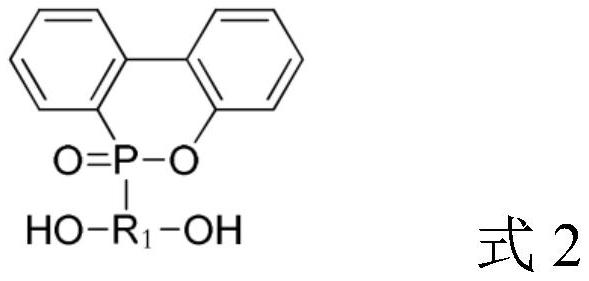
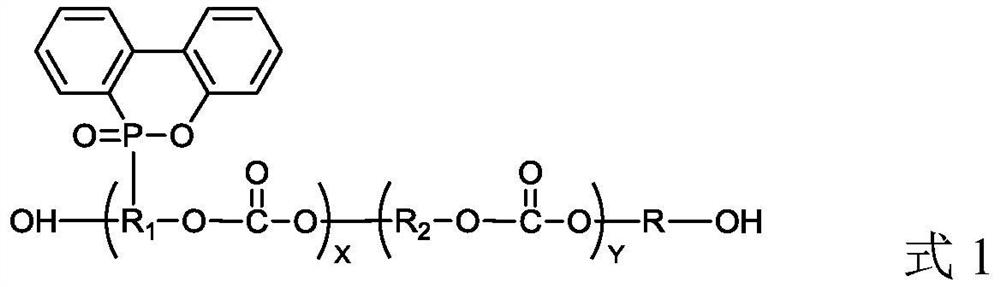
Polycarbonate polyol with phosphorus-containing side chain as well as preparation method and application of polycarbonate polyol
A polycarbonate and polyol technology, applied in the field of polycarbonate polyol and its preparation, can solve the problems of narrow molecular weight distribution, main chain phosphate is not resistant to hydrolysis, etc., and achieve narrow molecular weight distribution, excellent mechanical properties, and transparent color Odor free effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Add 232g of 9,10-dihydro-9-heterooxo-10-phosphophenanthrene-10-oxide (DOPO) and 72g of glycolaldehyde into a 5L reaction kettle, add 2200g of ethanol, heat up to 70°C, reflux conditions , stirred and reacted for 10 h, cooled and discharged, filtered, washed with ethanol, and dried at 100°C under nitrogen atmosphere to obtain intermediate A.
[0045] 13CNMR (CDCl 3 ,100MHz), δppm, 58.6, 81.1, 119.9, 121.1, 121.2, 121.8, 122.0, 125.0, 127.7, 129.0, 132.8, 136.4, 136.6, 150.2.
[0046](2) Under the protection of nitrogen, 72.7g of intermediate A, 279.7g of 1,6-hexanediol, 0.41g of tetraethyl titanate, and 181.2g of dimethyl carbonate were sent into a reaction kettle with a rectification tower, and the reaction The temperature of the kettle was gradually raised to 160°C, and the transesterification reaction was carried out at atmospheric pressure for 10 hours. At the same time, the rectification temperature at the top of the tower was controlled at 64°C, and by-product...
Embodiment 2
[0049] Add 232g of 9,10-dihydro-9-heterooxo-10-phosphinephenanthrene-10-oxide (DOPO) and 88g of 3-hydroxypropionaldehyde into a 5L reactor, add 2500g of toluene, heat up to 100°C, and reflux conditions , stirred and reacted for 8h, cooled and discharged, filtered, washed with toluene, and dried at 120°C under nitrogen atmosphere to obtain intermediate A.
[0050] Under the protection of nitrogen, 87.9g of intermediate A, 253.8g of 1,5-pentanediol, 0.45g of tetrapropyl carbonate, and 261.9g of diethyl carbonate were sent into the reaction kettle with a rectification tower, and the temperature of the reaction kettle was gradually raised to 170°C, transesterification reaction at normal pressure for 9 hours, while controlling the rectification temperature at the top of the tower to 78.3°C, and extract by-products at the top of the tower. After the transesterification reaction reached the end point, the pressure was reduced to 2.4kPa, vacuum polycondensation was carried out for 42 ...
Embodiment 3
[0052] Add 232g of 9,10-dihydro-9-heterooxo-10-phosphinephenanthrene-10-oxide (DOPO) and 105g of 4-hydroxybutyraldehyde into a 5L reactor, add 2300g of xylene, heat up to 130°C, and reflux Under the conditions, the reaction was stirred for 6 hours, cooled and discharged, filtered, washed with ethanol, and dried at 100°C under nitrogen atmosphere to obtain intermediate A.
[0053] Under the protection of nitrogen, 92.9g of intermediate A, 133.2g of 1,6-hexanediol, 117.4g of 1,5-pentanediol, 0.48g of dibutyltin dilaurate, and 314.5g of dipropyl carbonate were sent to the belt rectification In the reaction kettle of the tower, the temperature of the reaction kettle was gradually raised to 180°C, and the transesterification reaction was carried out at normal pressure for 8 hours. At the same time, the rectification temperature at the top of the tower was controlled at 97.2°C, and by-products were extracted from the top of the tower. After the transesterification reaction reaches t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com