Lithium ion battery positive electrode material and preparation method thereof
A technology for lithium ion batteries and cathode materials, applied in the field of new energy materials and their preparation, can solve the problems that the rate performance and structural stability of lithium iron manganese phosphate cannot be fully improved, and the interaction and affinity of lithium iron manganese phosphate are poor. The effect of improving ion and electron transport ability, enriching surface functional groups, and improving structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
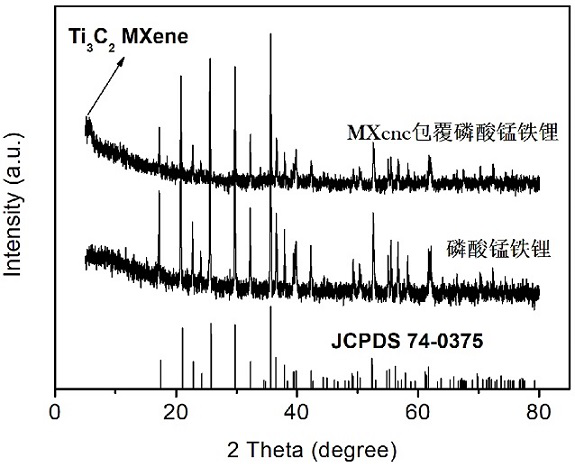
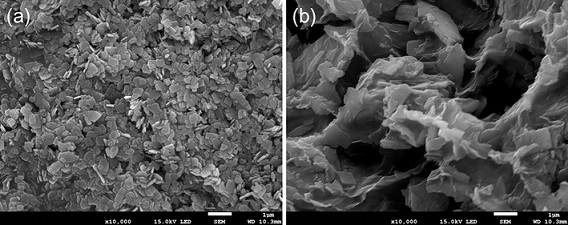
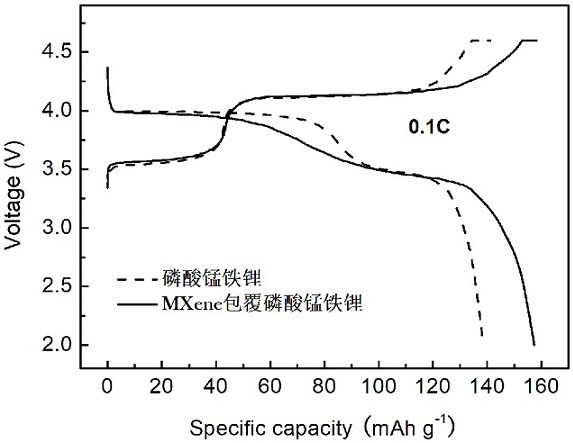
[0023] The Ti of lithium ion battery cathode material of the present invention 3 C 2 The preparation method of MXene-coated manganese iron phosphate material is to add phosphorus source and lithium source to deionized water / PEG solution to form suspension A, manganese source, iron source, antioxidant and Ti 3 C 2MXene is added to deionized water to form suspension B, and under continuous stirring conditions, suspension B is added dropwise to suspension A to form a mixed solution, and then the mixed solution is transferred to a hydrothermal reaction kettle and kept at a certain temperature for a period of time. After the reaction is completed, the product is separated by centrifugation, washed and dried, and finally the dried product is sintered in an atmosphere furnace to obtain Ti 3 C 2 MXene coated lithium manganese iron phosphate material. The method includes the following specific steps:
[0024] (1) Add phosphorus source and lithium source to deionized water / PEG sol...
Embodiment 1
[0038] Phosphoric acid and lithium hydroxide were added to the deionized water / PEG solution with a volume ratio of 2:1 to form suspension A, followed by manganese sulfate, ferrous sulfate, ascorbic acid and Ti 3 C 2 MXene is added to deionized water to form a suspension B, and under continuous stirring, the suspension B is added dropwise to the suspension A to form a mixed solution, wherein the element molar ratio Li of lithium source, manganese source, iron source and phosphorus source: Mn: Fe:P =3:0.8:0.2:2; Transfer the obtained mixture into a hydrothermal reactor, tighten the reactor and place it in an oven at 180°C for 10 hours. After the reaction is completed, centrifuge to separate the hydrothermal Product and it is dried under vacuum condition at 60 ℃ after washing; 2 Anneal at 650°C for 10 hours under atmosphere, and after the annealing is completed, black powder Ti 3 C 2 MXene coated lithium manganese iron phosphate material; Ti in this product 3 C 2 The cont...
Embodiment 2
[0040] Phosphoric acid and lithium hydroxide were added to the deionized water / PEG solution with a volume ratio of 2:1 to form suspension A, followed by manganese carbonate, ferrous chloride, ascorbic acid and Ti 3 C 2 MXene is added to deionized water to form a suspension B, and under continuous stirring, the suspension B is added dropwise to the suspension A to form a mixed solution, wherein the element molar ratio Li of lithium source, manganese source, iron source and phosphorus source: Mn: Fe:P = 3:0.5:0.5:2; transfer the obtained mixture into a hydrothermal reaction kettle, tighten the reaction kettle and place it in an oven at 140°C for 20h, and centrifuge to separate the hydrothermal reaction after the reaction is completed. Product and it is dried under vacuum condition at 80 ℃ after washing; 2 Anneal at 500°C for 20 hours in the atmosphere. After the annealing is completed, black powder Ti can be obtained. 3 C 2 MXene coated lithium manganese iron phosphate mate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com