Low-temperature copper-based core-shell catalyst for preparing methanol through CO2 hydrogenation and preparation method thereof
A core-shell catalyst and methanol production technology, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, hydroxyl compound preparation, etc., which can solve the cumbersome catalyst preparation process, lower reaction temperature, and lower conversion rate and other problems, to achieve excellent catalytic activity, high specific surface area/volume ratio, and promote the effect of binding strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
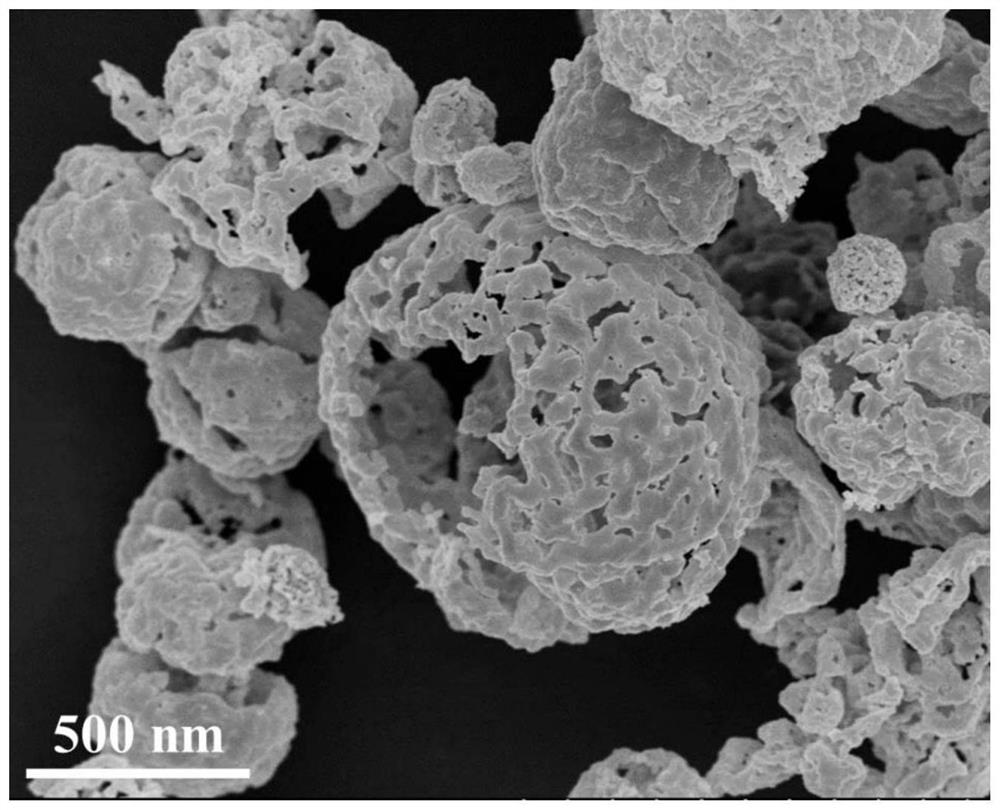
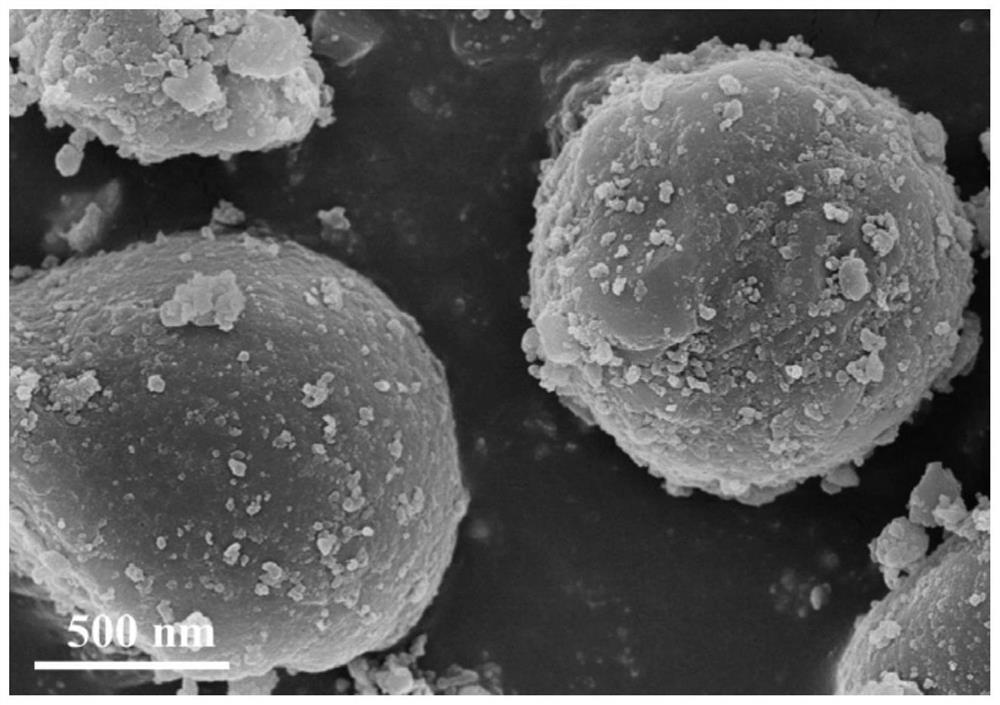
[0043] According to the molar ratio of copper nitrate, zinc nitrate and urea is 5.34:2.13:74.27, in a 500mL beaker, 20.0gD-(+)-glucose, 1.289g copper nitrate and 0.635g zinc nitrate were added to 170mL deionized water, at room temperature Stir magnetically until complete dissolution forms a mixed solution. Then 4.486g of urea was added into the mixed solution and stirred until completely dissolved to form a mixed solution. Next, the mixed solution was added into a 100 mL hydrothermal kettle (lined with polytetrafluoroethylene), and hydrothermally reacted at 160° C. for 7 h. After the reaction, cool down to room temperature naturally, then take out the dark brown product from the hydrothermal kettle, filter it with suction, and wash it alternately with ethanol and water for 3 to 5 times, then dry it in an oven at 100°C for 12 hours, and then place it in a muffle furnace. Calcined at 500°C for 6 hours to obtain copper-zinc oxide hollow spheres.
Embodiment 2
[0045] This embodiment is basically the same as Embodiment 1, the only difference is that the molar ratio of copper nitrate, zinc nitrate and urea is 7.35:2.94:102.42. 1.289g copper nitrate, 0.635g zinc nitrate and 4.486g urea in embodiment 1 are changed into 1.776g copper nitrate, 0.876g zinc nitrate and 6.186g urea respectively, all the other steps are with embodiment 1.
Embodiment 3
[0047] This embodiment is basically the same as Embodiment 1, the only difference is that the molar ratio of copper nitrate, zinc nitrate and urea is 9.36:3.75:130.30. 1.289g copper nitrate, 0.635g zinc nitrate and 4.486g urea in embodiment 1 are changed into 2.262g copper nitrate, 1.115g zinc nitrate and 7.870g urea respectively, all the other steps are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com