Modified lignin polyol and preparation method thereof
A polyol and lignin technology, applied in the field of modified lignin polyester polyol and its preparation, can solve the problems of catalyst toxicity, easy residue, and low material performance, and achieve low price, simple preparation, and high catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
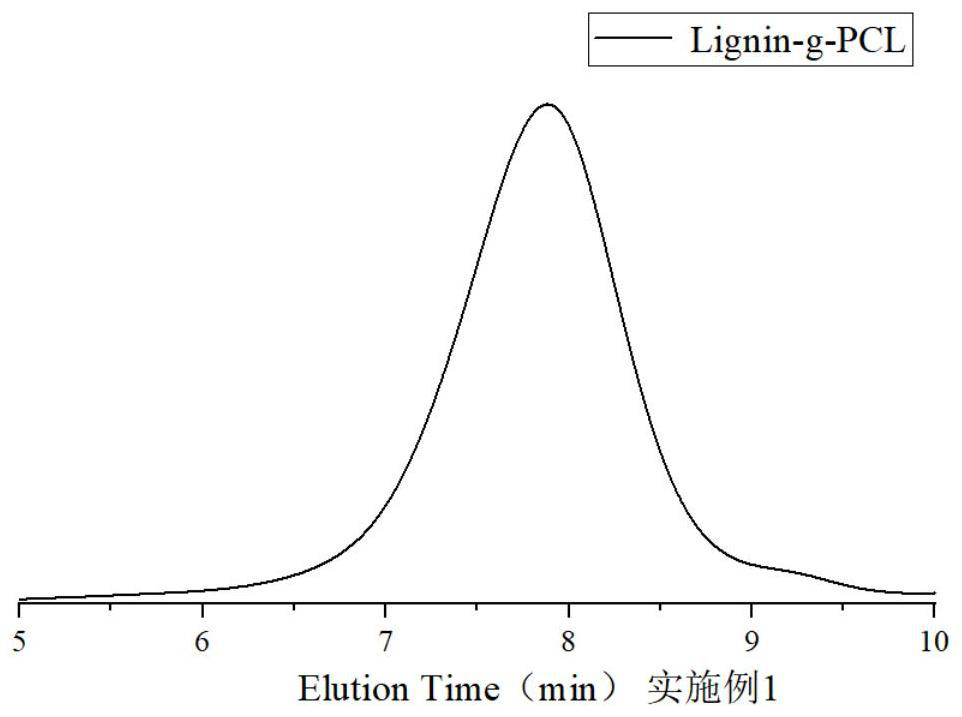
Image
Examples
Embodiment 1
[0058] The first step: mix the caprolactone monomer with calcium hydride, stir overnight to remove residual moisture, and purify by distillation under reduced pressure; place the lignin in a vacuum oven overnight to remove water; place the catalyst in a vacuum oven for use; react The required organic solvent is dewatered through a solvent redistillation device.
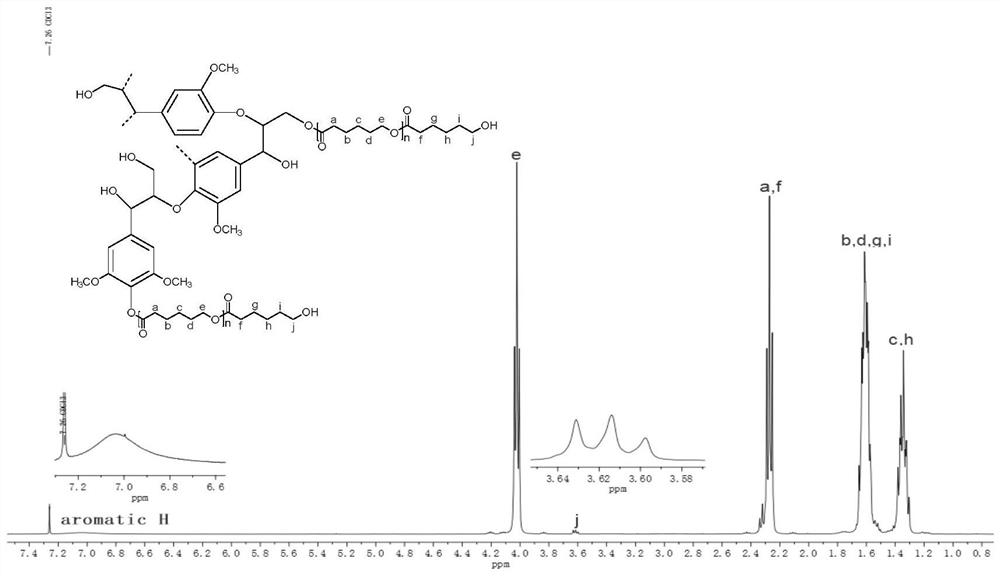
[0059] Step 2: Weigh caprolactone (1.80g), alkali lignin (0.20g) and dissolve in N,N-dimethylformamide (10mL) to obtain a homogeneous solution, add To the reaction flask, weigh o-vanillin Schiff base Ni (Ⅲ) catalyst (0.01g) and add to the reaction flask. Stir (500rpm) at 120°C for 3h. After the end, expose the reaction mixture to the air and let it stand for 30min. After the volatile matter is removed, the quenching is completed. Take a small part for 1 H NMR analysis (CDCl 3 as a deuterated reagent). The remaining part was dissolved in 15 mL of dichloromethane and centrifuged (3000 rpm for 3 min). The supernatant...
Embodiment 2
[0061] The first step: mix the caprolactone monomer with calcium hydride, stir overnight to remove residual moisture, and purify by distillation under reduced pressure; place the lignin in a vacuum oven overnight to remove water; place the catalyst in a vacuum oven for use; react The required organic solvent is dewatered through a solvent redistillation device.
[0062] Step 2: Weigh caprolactone (1.90g), alkali lignin (0.10g) and dissolve in N,N-dimethylformamide (10mL) to obtain a homogeneous solution. Into the reaction flask, weigh o-vanillin Schiff base Ni (Ⅲ) catalyst (0.02g) and add to the reaction flask. React at 120°C with a stirring rate of 500rpm for 1h. After the end, expose the reaction mixture to the air and let it stand for 30min. After removing the volatile matter, the quenching is completed. Take a small part for further analysis. 1 H NMR analysis (CDCl 3 as a deuterated reagent). The remaining part was dissolved in 15 mL of dichloromethane and centrifuged (...
Embodiment 3
[0064] The first step: mix the caprolactone monomer with calcium hydride, stir overnight to remove residual moisture, and purify by distillation under reduced pressure; place the lignin in a vacuum oven overnight to remove water; place the catalyst in a vacuum oven for use; react The required organic solvent is dewatered through a solvent redistillation device.
[0065] Step 2: Weigh caprolactone (1.70g), alkali lignin (0.30g) and tetrahydrofuran (10mL) and mix them, add them to the reaction bottle in sequence with a magnetic stirrer under nitrogen protection, and weigh o-vanillin mat Ni(Ⅲ) catalyst (0.01g) was added to the reaction flask and stirred (500rpm) at 100°C for 6h. After the end, the reaction mixture was exposed to the air and left for 30min. After the volatile matter was removed, the quenching was completed. Take a small in part 1 H NMR analysis (CDCl 3 is a deuterated reagent). The remaining part was dissolved in 15 mL of dichloromethane and centrifuged (3000 r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com