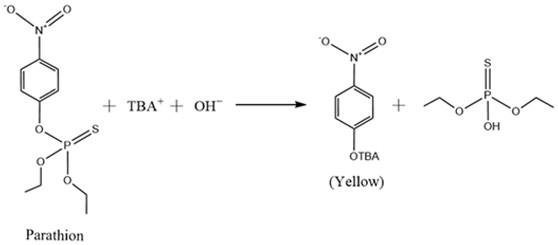
Method for determining parathion in grain based on dispersive liquid-liquid microextraction of hydrophilic and hydrophobic deep-eutectic solvent in combination with digital image colorimetric method
A low eutectic solvent, liquid dispersion technology, applied in material analysis by observing the effect of chemical indicators, preparation of test samples, analysis by chemical reaction of materials, etc., can meet the professional requirements of technicians High, long detection time, expensive equipment and other problems, to avoid organic solvents, less sample required, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: verification of experimental feasibility
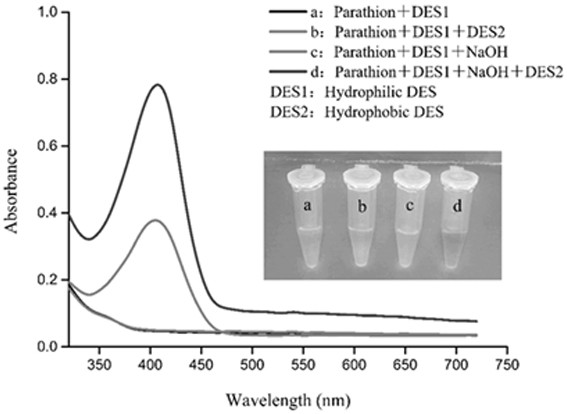
[0048] Preparation method: Prepare four 1.5 mL centrifuge tubes and configure four different reaction systems: a: parathion + hydrophilic DES; b: parathion + hydrophilic DES + hydrophobic DES; c: parathion + hydrophilic DES+alkali; d: parathion+hydrophilic DES+alkali+hydrophobic DES; measure their UV absorption spectra respectively.
[0049] The experimental results are shown in Figure 3: when the hydrophobic DES and sodium hydroxide do not exist, the a system is colorless. When the hydrophobic DES exists and the sodium hydroxide does not exist, the b system has no obvious change, and the solution is still colorless. When sodium hydroxide exists and hydrophobic DES does not exist, the c system has a weak absorption peak around 410 nm, but the solution is still colorless. When the hydrophobic DES and sodium hydroxide were present at the same time, a significant absorption peak appeared in the d system at around 4...
Embodiment 2
[0050] Example 2: condition optimization
[0051] A. Types of hydrophilic DES
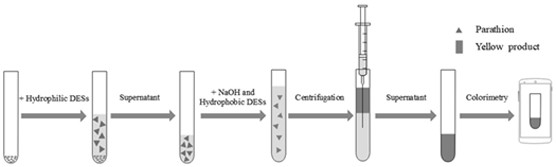
[0052] (1) Experimental method: Accurately weigh 0.1 g of sample into a 0.5 mL centrifuge tube, add 200 μL of hydrophilic DES (choline chloride is synthesized with ethylene glycol, propylene glycol, and butanediol, respectively), and then vortex for 60 s , put it in a centrifuge and centrifuge at 6000r / min for 1min, take out the supernatant and place it in a 0.5 mL centrifuge tube, add 100 μL sodium hydroxide aqueous solution, and then add 100 μL hydrophobic DES, hydrophobic DES can dissociate in situ, dissociated hydrophilic TBA + Under alkaline conditions, the parathion in the hydrophilic DES was converted into a yellow product, which was extracted by the hydrophobic extractant terpineol generated in situ and dispersed in the solution, and centrifuged at 6000r / min for 1min. Remove the lower aqueous phase with a syringe. Then take pictures of the colored part in the centrifuge tube, analyze the...
Embodiment 3
[0081] Embodiment 3: the making of parathion standard curve
[0082] (1) Experimental method: Accurately weigh 0.1 g of sample (addition concentration is 0.01 mg kg -1 , 0.05 mg kg -1 , 0.10 mg kg -1 , 0.50 mg kg -1 , 1.00 mg kg -1 , 2.00 mg kg -1 , 5.00 mg kg -1), add 200 μL of hydrophilic DES (choline chloride and propylene glycol are synthesized by 1 / 3), then vortex extract for 60 s, put it in a centrifuge and centrifuge at 3260 g for 1 min, take out the supernatant and place it in a 0.5 mL centrifuge Add 100 μL sodium hydroxide aqueous solution to the tube, then add 100 μL hydrophobic DES (tetrabutylammonium bromide and terpineol are synthesized by 1 / 1), centrifuge at 3260 g for 1 min, dissolve parathion in pine oil The alcoholic organic phase is located in the upper layer of the centrifuge tube, and the lower aqueous phase is removed with a syringe. Then take pictures of the colored part in the centrifuge tube, and analyze and calculate the RGB value (I). Draw the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com