Organic chlorine removal catalyst and preparation method and application thereof
A technology for removing organochlorine and catalysts, which is applied in the field of organochlorine removal catalysts and its preparation, can solve the problems of undisclosed and more scientific removal, achieve long service life, high removal precision, and reduce the effect of total chlorine at the outlet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) 10g of activated alumina, 65g of ZSM-5 molecular sieve, 15g of coconut shell activated carbon, and 10g of magnesium aluminum hydrotalcite are mixed evenly and then ground into particles with a particle size of less than 1000 mesh;
[0036] (2) Add an appropriate amount of scallop powder, water, and sodium hydroxide solution to the mixture particles obtained in step (1), knead after mixing, and extrude into strips with a diameter of 3-5mm, dry at 120°C for 4 hours, and then dry at 500°C ℃ roasting for 4 hours to obtain the deorganochlorination catalyst TL-1.
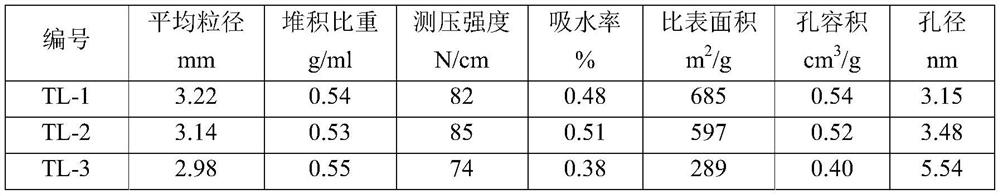
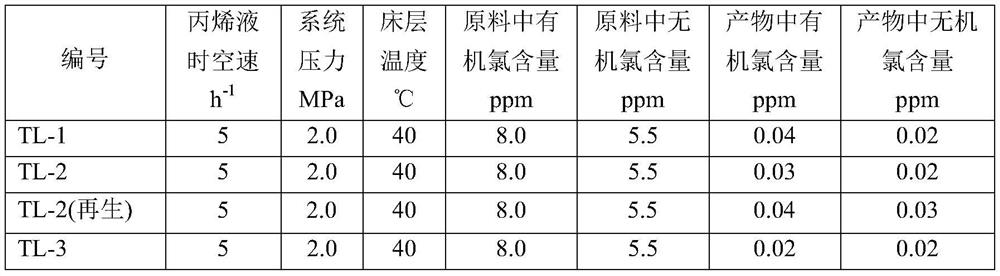
[0037] (3) Analyze physical properties such as bulk specific gravity, piezometric strength, water absorption, X-ray fluorescence spectrum, physical adsorption, and X-ray diffraction of the prepared organochlorine removal agent, and test the physical property indicators of the prepared organochlorine removal catalyst. The physical data are shown in Table 1. It was then placed in a fixed-bed micro-reaction evalu...
Embodiment 2
[0039] (1) 5g of activated alumina, 75g of ZSM-5 molecular sieve, 10g of coconut shell activated carbon, and 10g of magnesium aluminum hydrotalcite are mixed evenly and then ground into particles with a particle size of less than 1000 mesh;
[0040] (2) Add an appropriate amount of carboxymethyl cellulose and 3% dilute nitric acid, cesium nitrate, copper nitrate solution to the mixture particles obtained in step (1), knead after mixing, extrude and form, and the diameter is 3-5mm. Dry at 120°C for 2 hours, and then bake at 500°C for 4 hours to obtain the catalyst for removing organochlorine TL-2.
[0041] (3) Analyze physical properties such as bulk specific gravity, piezometric strength, water absorption, X-ray fluorescence spectrum, physical adsorption, and X-ray diffraction of the prepared organochlorine removal agent, and test the physical property indicators of the prepared organochlorine removal catalyst. The physical data are shown in Table 1. It was then placed in a f...
Embodiment 3
[0044] (1) 30g of activated alumina, 45g of ZSM-35 molecular sieve, 20g of coconut shell activated carbon, and 5g of magnesium aluminum hydrotalcite are mixed evenly and then ground into particles with a particle size of less than 1000 mesh;
[0045] (2) Add an appropriate amount of carboxymethyl cellulose and 3% dilute nitric acid and cerium nitrate solution to the mixture particles obtained in step (1), knead after mixing, extrude and form, with a diameter of 3-5mm, and dry at 120°C 2h, and then calcined at 500°C for 2h to obtain organochlorine removal catalyst TL-3.
[0046] (3) Analyze physical properties such as bulk specific gravity, piezometric strength, water absorption, X-ray fluorescence spectrum, physical adsorption, and X-ray diffraction of the prepared organochlorine removal agent, and test the physical property indicators of the prepared organochlorine removal catalyst. The physical data are shown in Table 1. It was then placed in a fixed-bed micro-reaction eval...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com