Method for regenerating iron phosphate from phosphorus iron slag after lithium extraction
A technology of iron phosphate and ferrophosphorus slag is applied in the field of waste resource recycling and lithium ion battery materials, which can solve the problems of alkali consumption and acid consumption, achieve high recovery rate, reduce acid and alkali consumption, and low-value by-products less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
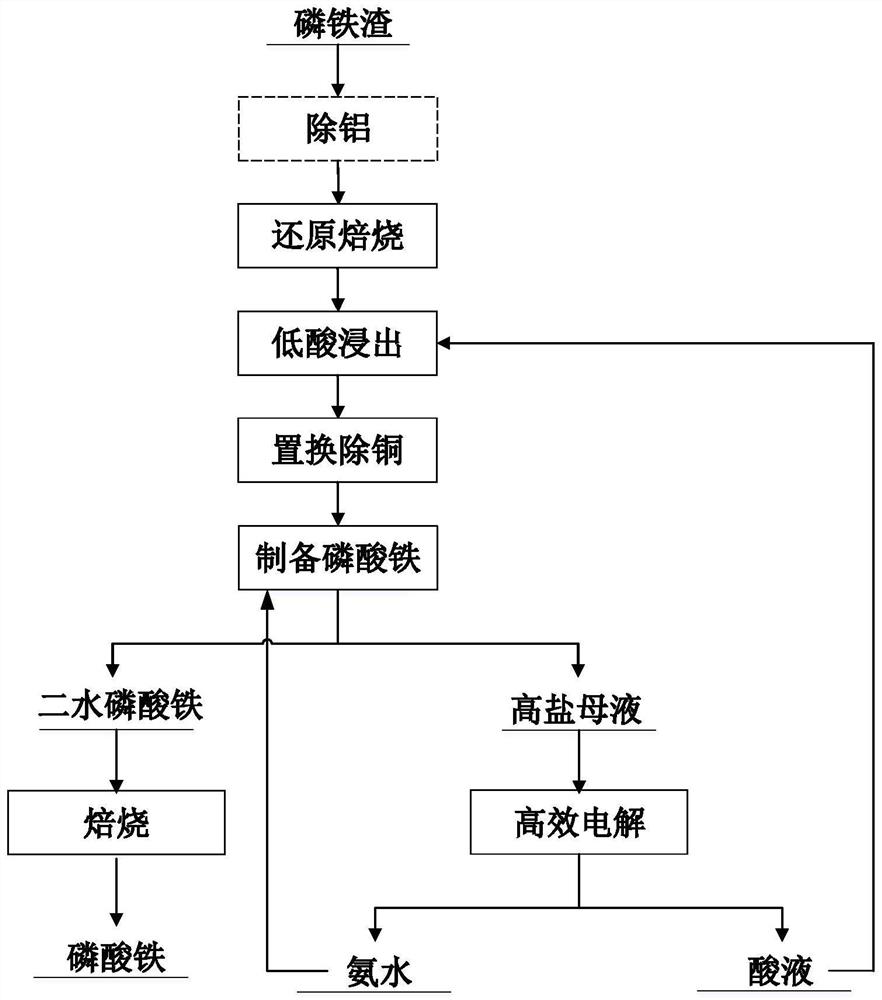
[0032] A method for regenerating iron phosphate slag from lithium after lithium extraction, the method may further comprise the steps:
[0033] Mix the ferrophosphorus slag after lithium extraction with pure water to make slurry according to the solid-to-liquid ratio of 1:5, add sodium hydroxide solution twice the theoretical amount to the slurry, react for 1 hour, filter and wash the solid to obtain slag A.
[0034] After drying the slag A in a vacuum drying oven, add 2 times the theoretical amount of glucose to mix evenly, put it into a tube furnace and roast it for 2 hours to obtain the slag B.
[0035] Slag B and dilute phosphoric acid solution were slurried at a mass ratio of 1:6, stirred and leached at 80°C for 2 hours to obtain solution A, and the phosphorus-iron ratio was adjusted to 1.1:1 to obtain solution B.
[0036] At 60°C, add 6vol% hydrogen peroxide dropwise to solution B, use ammonia water to adjust the pH value of the solution to 2 for co-precipitation, and ob...
Embodiment 2
[0039] A method for regenerating iron phosphate slag from lithium after lithium extraction, the method may further comprise the steps:
[0040] The ferrophosphorus slag after lithium extraction was mixed with 2 times the theoretical amount of charcoal powder and roasted in a tube furnace for two hours to obtain slag A.
[0041] Slag A and water were slurried at a mass ratio of 1:3, sulfuric acid was added, and solution A was obtained by leaching at 25°C for 2 hours.
[0042] Add iron powder twice the amount of theory to solution A to replace and remove copper, and filter to obtain solution B.
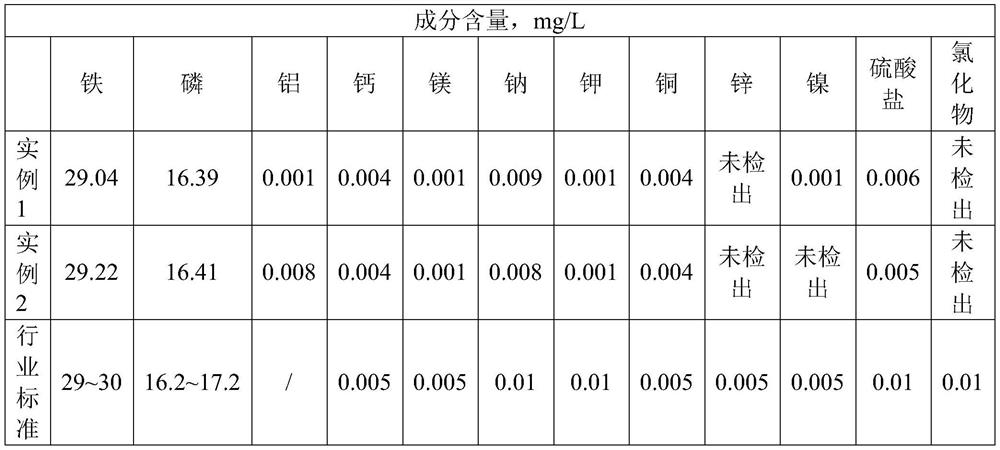
[0043] Add ammonium dihydrogen phosphate to adjust the phosphorus-iron ratio to 1:1, then add hydrogen peroxide and ammonia water for co-precipitation, and obtain ferric phosphate dihydrate and precipitated liquid after solid-liquid separation, in which ferric phosphate dihydrate is roasted in a muffle furnace at 600 °C Ferric phosphate was obtained in 4 hours, and the solution after p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com