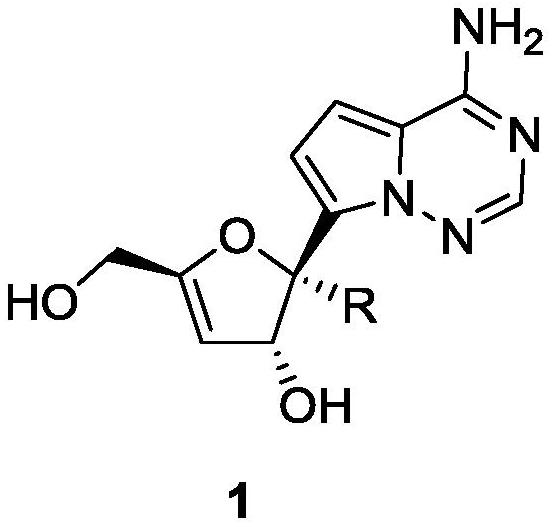
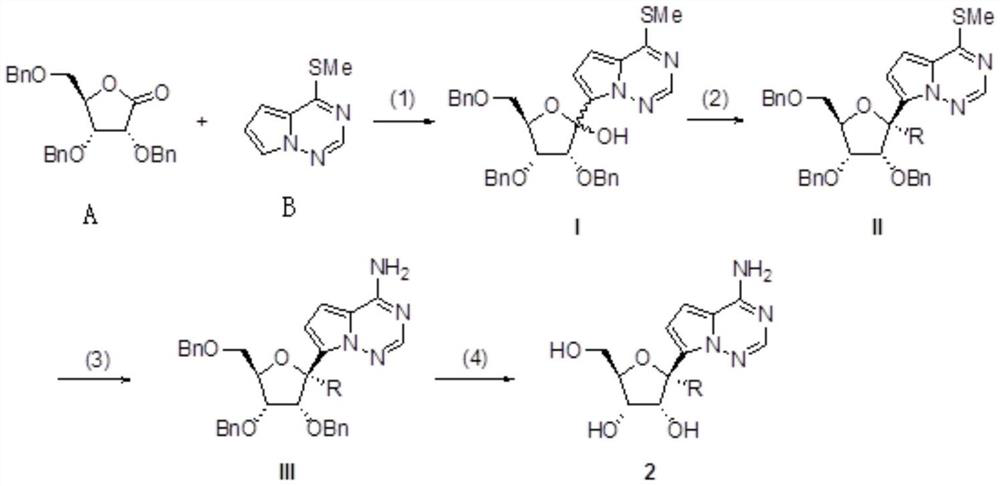
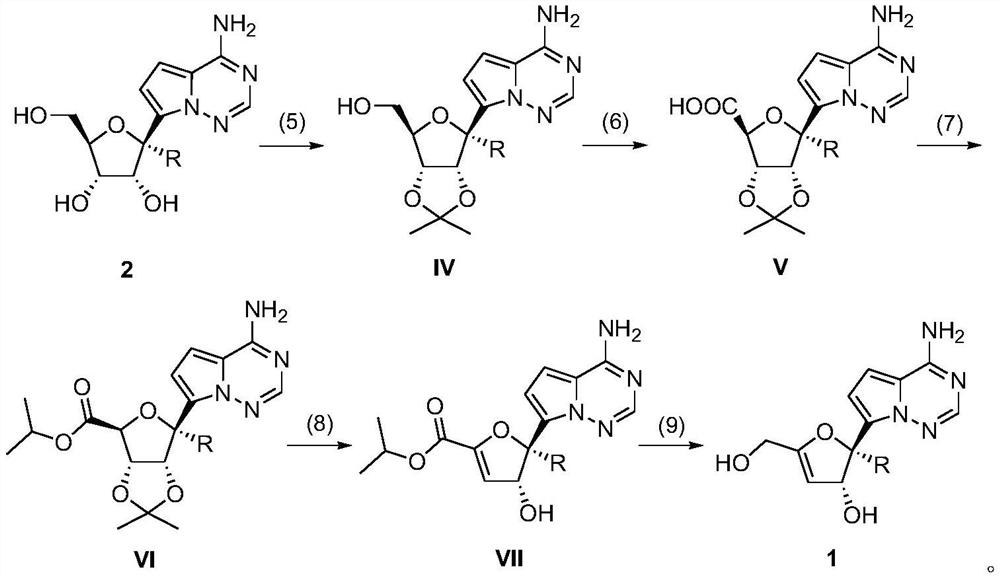
3 ', 4'-unsaturated ribose C-nucleoside analogue and preparation method thereof
A technology for nucleoside analogs and ribonucleosides, which is applied in the field of novel 3',4'-unsaturated-C-ribonucleoside analogs and their preparations, and in the field of antiviral activity, and can solve the problems of insufficient activity to make medicines, etc. Achieve good development prospects, strong stability, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of ribose C-nucleoside (general formula 2, R=H, CN)
[0040] Dissolve 4-aza-7,9-dideaza-6-methylthioadenine (1g, 6.05mmol) in dry tetrahydrofuran (25mL), add 2M diisopropylamino at -78°C under nitrogen protection Lithium tetrahydrofuran solution (4.5mL, 9.08mmol), after reacting for 30min, add 2,3,5-tri-O-benzylribonolactone (3.04g, 7.26mmol) in tetrahydrofuran (10mL) solution, continue the reaction at -78°C After 1 h, it was quenched by adding saturated ammonium chloride solution, extracted with ethyl acetate, and the organic phase was washed with brine, dried over anhydrous sodium sulfate, filtered, and concentrated. Silica gel column chromatography (petroleum ether: ethyl acetate volume ratio = 20:1 ~ 5:1) gave oily liquid I (a mixture of α and β configurations).
[0041] Compound I (1.0 g, 1.71 mmol) was dissolved in dichloromethane, under nitrogen protection, triethylsilane (1.13 mL, 6.85 mmol) and boron trifluoride ether solution (430 μL, 3...
Embodiment 2
[0047] Embodiment 2: preparation compound 1a (general formula 1, R=H)
[0048] Suspend compound 2a (0.5g, 1.75mmol) in acetone (37mL, 500mmol), add perchloric acid (225μL, 3.94mmol) and 2,2-dimethoxypropane (216μL, 1.75mmol), stir at room temperature, After the reaction was detected by TCL, it was quenched by adding ammonia water, and the volatile matter was distilled off under reduced pressure. Separation by silica gel column chromatography (dichloromethane: methanol volume ratio = 10:1), rotary evaporation to obtain oily liquid IVa.
[0049] Ⅳa (1g, 3.07mmol), TEMPO (240mg, 1.54mmol), BAIB (3.96g, 12.3mmol) were successively added to the reaction flask, and then acetonitrile / water (V / V=1 / 1, 40mL) was added, and the Stirring, TCL detects that after the reaction is complete, the volatile matter is distilled off under reduced pressure. Acetone and ether were added for recrystallization and filtration to obtain Va as a white solid.
[0050] Suspend Va (0.5g, 1.47mmol) in anhy...
Embodiment 3
[0053] 1 H NMR (400MHz, DMSO) δ7.87(s, 1H), 7.74(s, 2H), 6.84(d, J=4.4Hz, 1H), 6.58(d, J=4.4Hz, 1H), 5.61(d ,J=3.1Hz,1H),5.30(d,J=5.9Hz,1H),5.15(t,J=5.9Hz,1H),5.03(d,J=4.8Hz,2H),3.99–3.93(m , 2H). Embodiment 3: Preparation of compound 1b (general formula 1, R=CN)
[0054] Using the same method as in Example 2, using 2b as a raw material, tetramethylethylenediamine was added to quench after the reduction, and a white solid 1b was obtained.
[0055] 1 H NMR (400MHz, DMSO) δ7.95(d, J=19.7Hz, 3H), 6.91(d, J=4.5Hz, 1H), 6.79(d, J=4.5Hz, 1H), 6.33(d, J =7.0Hz, 1H), 5.36(t, J=5.9Hz, 1H), 5.22(d, J=6.8Hz, 1H), 5.12(s, 1H), 4.07(d, J=5.7Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com