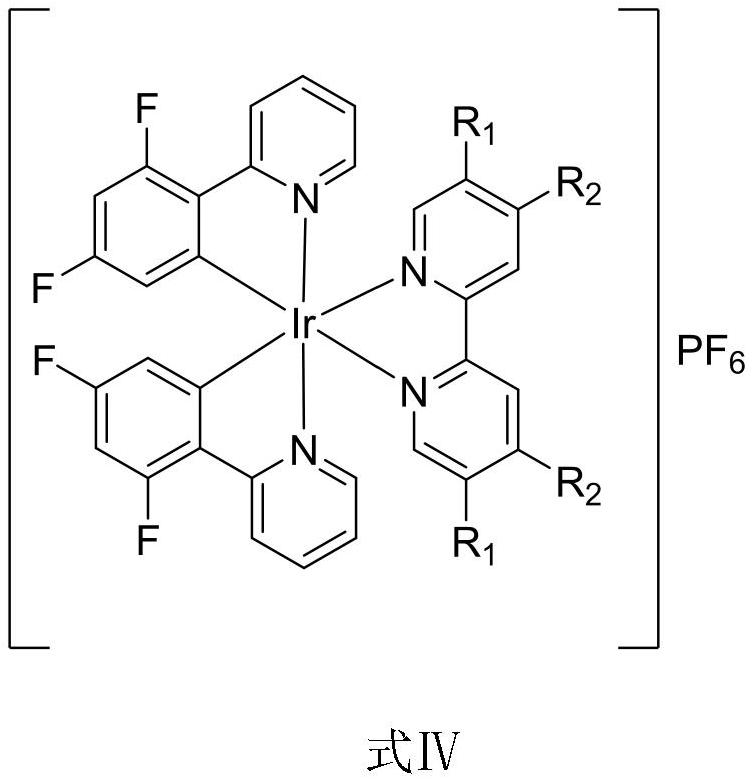
Fluorine-containing metal iridium complex as well as synthesis method and application thereof
A technology of metal iridium and complexes, which is applied in the field of fluorine-containing metal iridium complexes and their synthesis, can solve the problems of single iridium complexes, poor performance, unfavorable promotion, etc., and achieve low synthesis cost and easy operation. Simple, highly catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Preparation of Intermediate I
[0034]
[0035] Under nitrogen protection, 0.58mol of 2-bromopyridine, 0.6mol of 2,4-difluorophenylboronic acid, 1.35mmol of Pd(dppf)Cl 2, 0.87mol of sodium carbonate was added to the three-necked flask, and then a mixed solution (1.2L) of 1,4-dioxane and water was added, wherein the volume ratio of 1,4-dioxane to water was 5 :1; then the mixed reaction solution was heated to 70°C and refluxed for 1h. After the reaction was completed, it was cooled to room temperature, and the solvent was distilled off under reduced pressure. The obtained solid was separated and extracted with water and MTBE, and the organic phase was washed with saturated brine and water successively, dried over anhydrous sodium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain a white solid intermediate I with a yield of 90.8%.
[0036] 2) Preparation of Intermediate II
[0037]
[0038] Under nitrogen protection, 28mmol o...
Embodiment 2
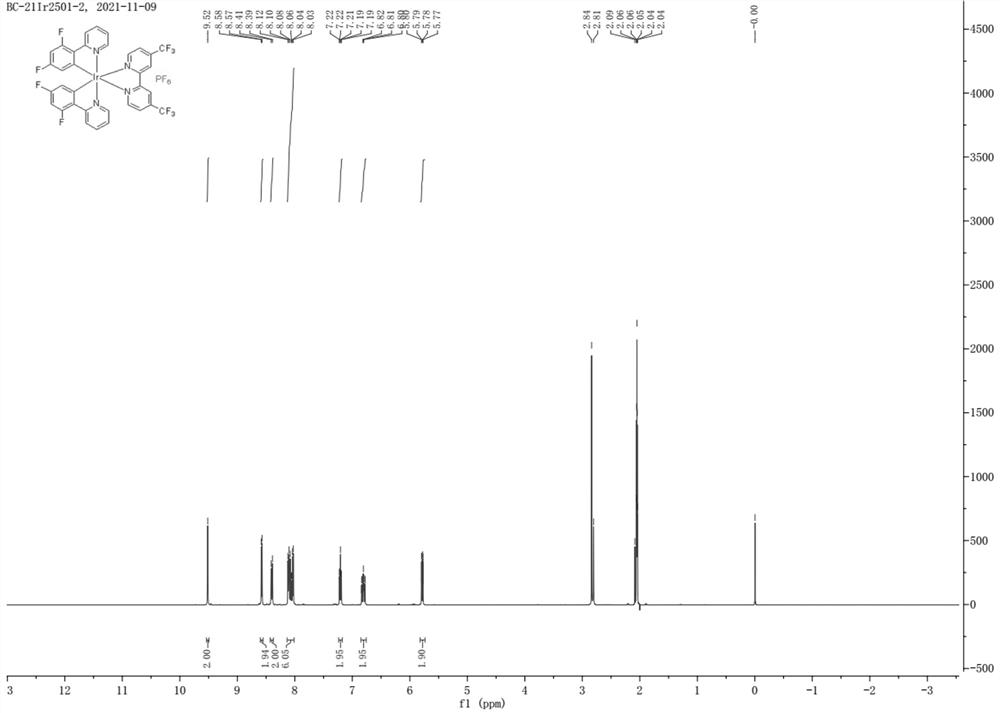
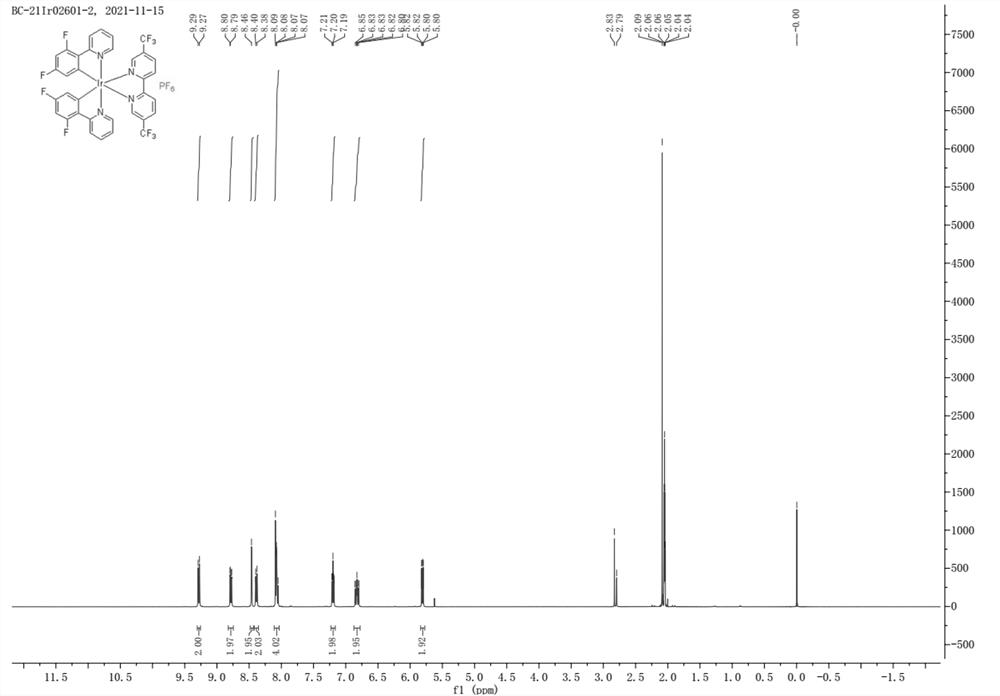
[0046] Example 2 The experimental process for preparing the iridium complex photocatalyst was repeated in Example 1, except that the 4,4'-bis(trifluoromethyl)-2,2'-bipyridine in step 3) was replaced by the same Molar amount of 5,5'-bis(trifluoromethyl)-2,2'-bipyridine, the rest of the operations were the same as in Example 1, and finally No. 2 novel iridium complex photocatalyst was obtained. The product was subjected to nuclear magnetic resonance detection, and the results were as follows: figure 2 shown.
Embodiment 3-5
[0052] The experimental process of embodiment 3-5 preparation iridium complex photocatalyst repeats embodiment 1, and difference only is that the proportioning of ethylene glycol methyl ether and water is different in step 2) (the ratio of ethylene glycol methyl ether and water mixed solution Total volume is constant 300mL), and all the other operations are with embodiment 1, and the intermediate II yield result that finally makes is as shown in table 2:
[0053] Table 2: Yields of products obtained with different ratios of ethylene glycol methyl ether and water
[0054]
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com