Compound containing 1, 3-diketone ligand, application of compound and organic electroluminescent device
A compound and ligand technology, applied in the field of organic electroluminescent devices, can solve the problems of low luminous efficiency and large efficiency roll-off, and achieve the effects of improving luminous efficiency, increasing lifespan, and reducing concentration quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1-3
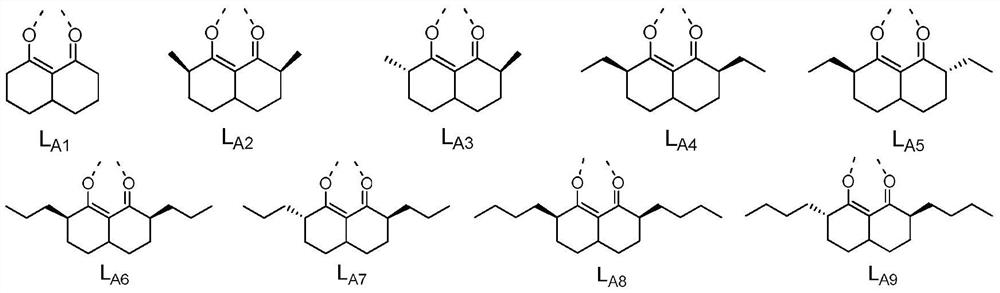
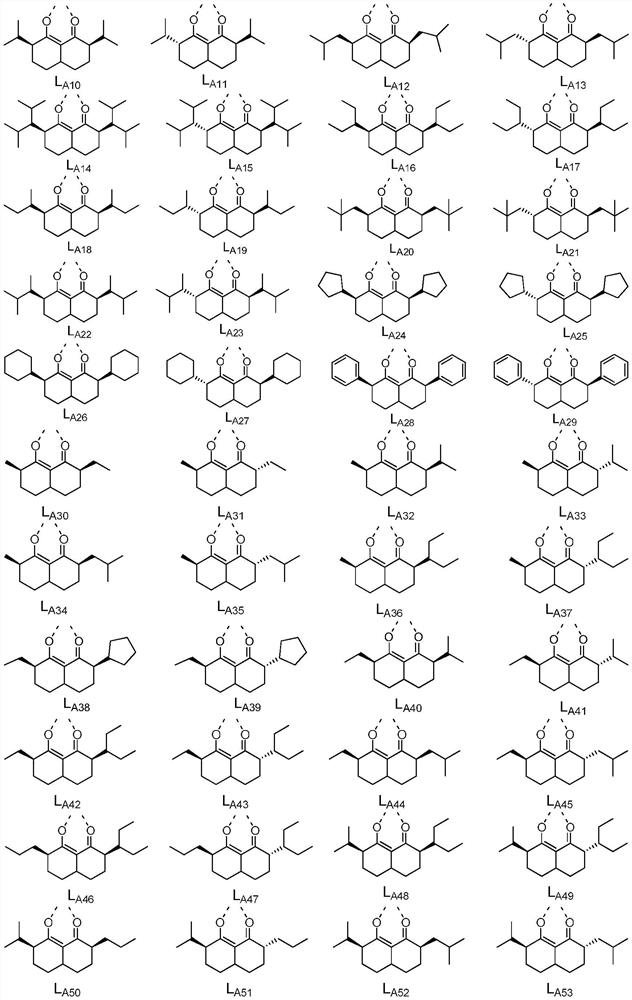
[0075] According to particularly preferred embodiments 1-3 , in Ir(L A )(L B ) 2 In the structure shown,
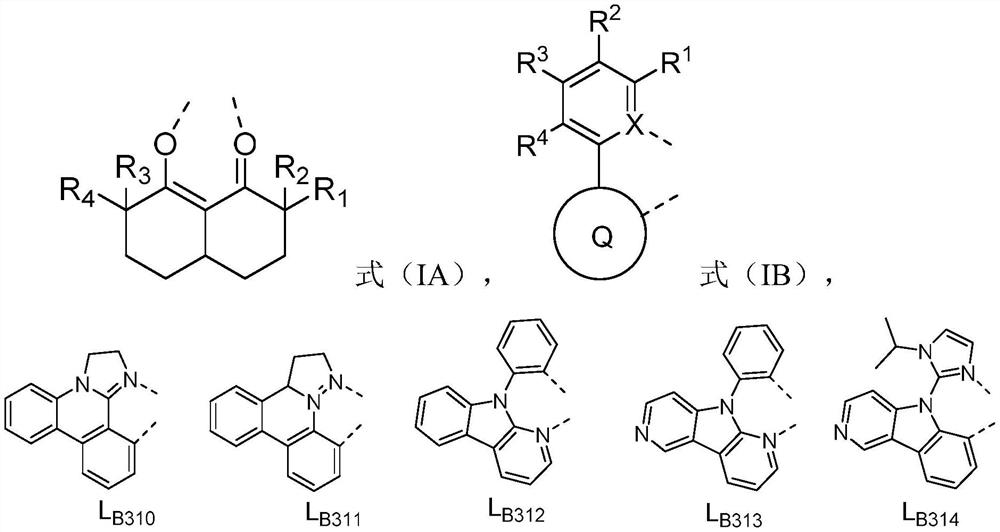
[0076] In formula (IA), R 1 , R 2 , R 3 , R 4 each independently selected from H, C 1- C 7 Alkyl, C 6- C 10 Aryl; or R 1 with R 2 combination of and R 3 with R 4 At least one combination of the combinations is ring-closed to form a 4-6 membered saturated ring;
[0077] In formula (IB), X is C or N,
[0078] Q ring is selected from substituted or unsubstituted benzene ring, substituted or unsubstituted quinoline ring, substituted or unsubstituted isoquinoline ring, substituted or unsubstituted naphthalene ring, substituted or unsubstituted phenanthrene ring, substituted or unsubstituted Substituted benzothiophene ring, substituted or unsubstituted benzofuran ring, substituted or unsubstituted indole ring, substituted or unsubstituted benzothiazole ring, substituted or unsubstituted benzoxazole ring, substituted or unsubstituted Substituted benzimidazole r...
specific Embodiment approach 1-4
[0083] According to particularly preferred embodiments 1-4 , in Ir(L A )(L B ) 2 In the structure shown,
[0084] In formula (IA), R 1 , R 2 , R 3 , R 4 each independently selected from H, methyl, ethyl, C 3 straight chain alkyl, C 3 branched chain alkyl, C 3 Cycloalkyl, C 4 straight chain alkyl, C 4 branched chain alkyl, C 4 Cycloalkyl, C 5 straight chain alkyl, C 5 branched chain alkyl, C 5 Cycloalkyl, C6 straight chain alkyl, C 6 branched chain alkyl, C 6 Cycloalkyl, C 7 straight chain alkyl, C 7 branched chain alkyl, C 7 Cycloalkyl, phenyl; or R 1 with R 2 combination of and R 3 with R 4 At least one combination of the combinations is ring-closed to form a 4-6 membered saturated ring;
[0085] In formula (IB), X is C or N,
[0086] Q ring is selected from substituted or unsubstituted benzene ring, substituted or unsubstituted quinoline ring, substituted or unsubstituted isoquinoline ring, substituted or unsubstituted naphthalene ring, substituted ...
preparation example 1
[0132] Preparation Example 1: Compounds shown in the preparation formula M1
[0133]
[0134] Synthesis of intermediate M1-1: under nitrogen protection, dissolve the activated zinc powder (0.4mol) in 30ml of anhydrous THF, then add trimethylchlorosilane (25ml), stir for 15min, then add 4-iodobutyl Ethyl acetate (0.4mol), stirred at 30°C for 12h, cooled to -10°C, then added copper cyanide (0.2mol) and lithium chloride (0.4mol) in THF solvent (200ml), heated to 0°C , stirred for 10 min, and cooled to -78°C to obtain No. 1 solution.
[0135] 2-Cyclohexen-1-one (0.28mol) and trimethylchlorosilane (0.66mol) were dissolved in ether (250ml), then slowly added dropwise to No. 1 solution, stirred at -78°C for 3h, Warm up to room temperature and react for 12h. Add saturated NH 4 Cl (450ml) and saturated NH 4 OH (50ml) was used to quench the reaction, extracted three times with ethyl acetate, the organic phase was combined, the organic phase was removed by rotary evaporation, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com