Method for recycling valuable metal in waste ternary lithium ion battery
A technology for lithium-ion batteries and valuable metals, which is applied in the field of recycling valuable metals in waste ternary lithium-ion batteries, can solve the problems of inability to obtain pure products, difficult and complex waste treatment, and increased equipment investment, so as to achieve the same treatment cost Secondary pollution, wide application range and low treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
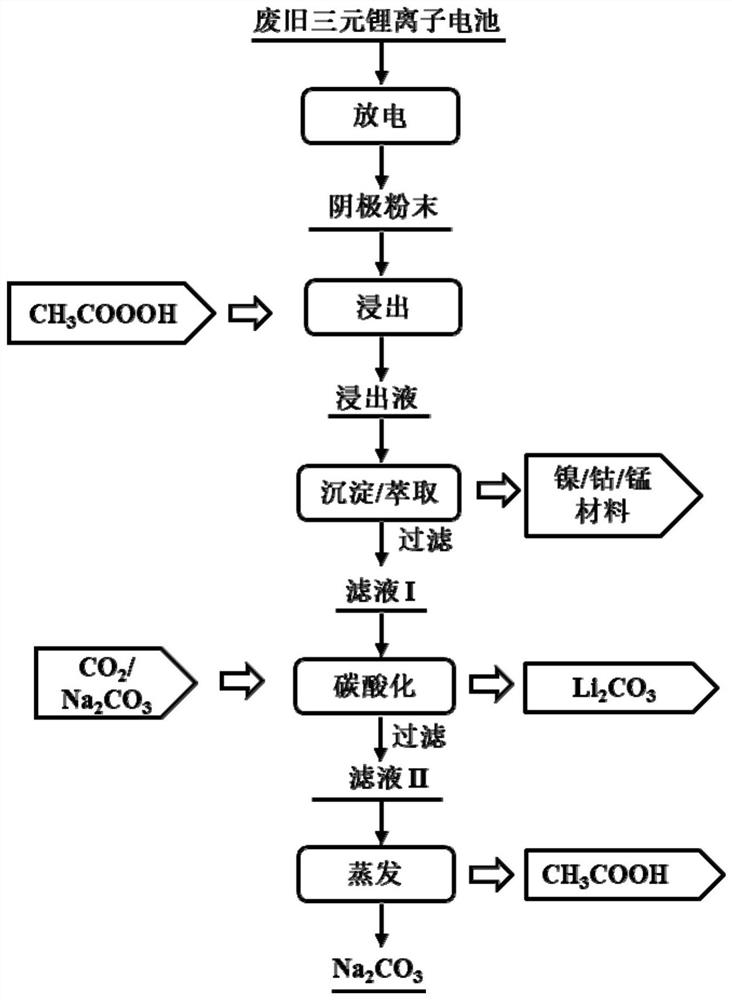
[0053] Embodiment 1 operation process such as figure 1 shown. details as follows:
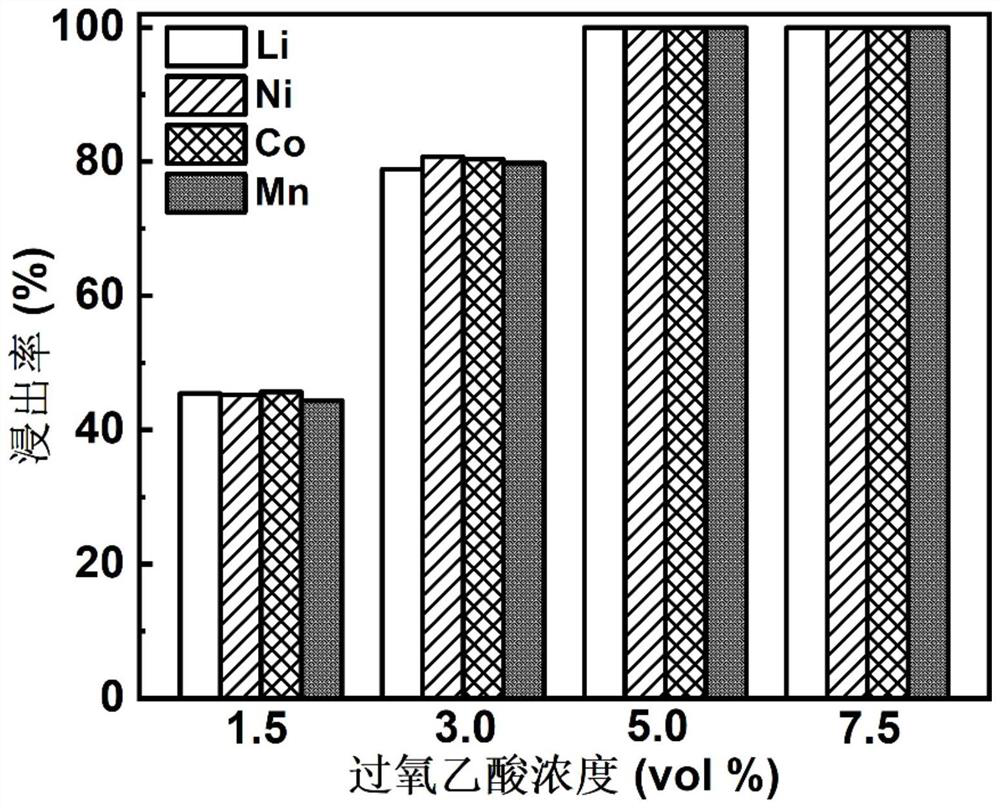
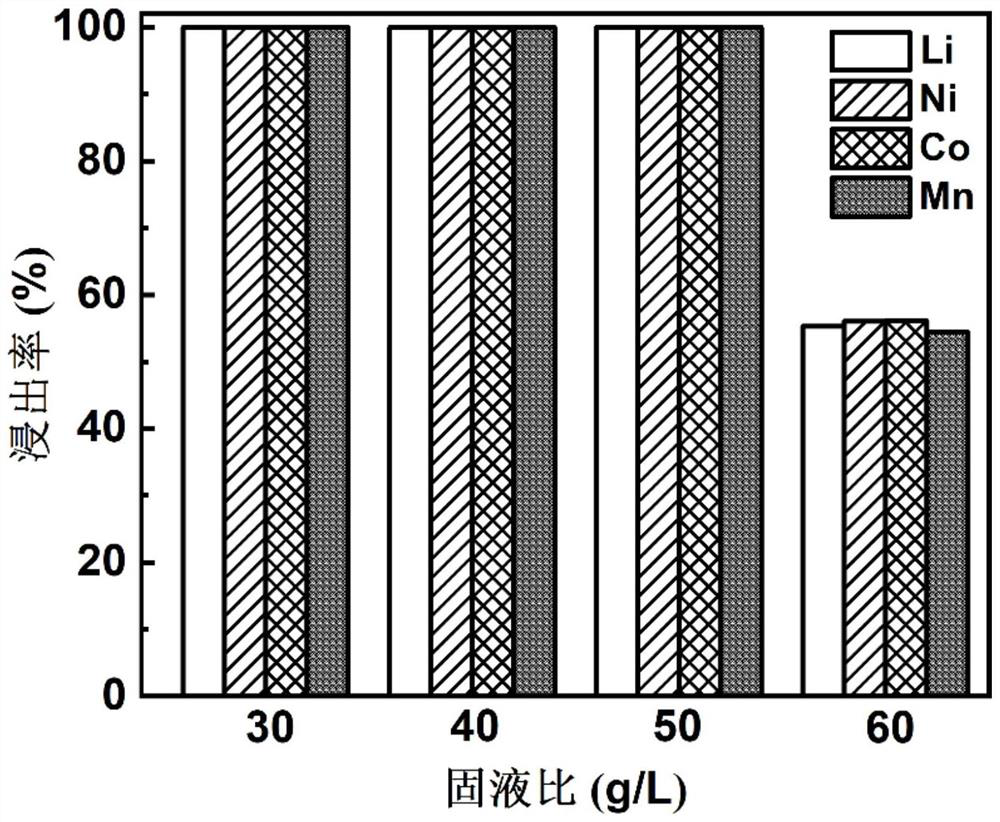
[0054] Discharge the waste 622-type nickel-cobalt-manganese ternary lithium-ion battery and disassemble it in a glove box to obtain NCM622 positive electrode powder; add 10mL peracetic acid solution dropwise at a rate of 2mL / min to a sample bottle containing 0.5g NCM622 positive electrode powder Among them, the volume concentration of peracetic acid in the peracetic acid solution is 5.0%, in addition, the reaction temperature is controlled by an oil bath to be 60°C, the rotating speed is controlled by magnetic stirring to be 600rpm, the reaction time is controlled to be 60min, and the solid-liquid ratio is controlled The content of lithium, nickel, cobalt, and manganese in the leaching solution was tested by ICP-MS (inductively coupled plasma mass spectrometry), and the leaching rates of lithium, nickel, cobalt, and manganese were 99.99%, 99.97%, 99.98%, and 99.99%, respectively. %.
[0055]...
Embodiment 2
[0057] Discharge the waste 622-type nickel-cobalt-manganese ternary lithium-ion battery and disassemble it in a glove box to obtain NCM622 positive electrode powder; add 10mL peracetic acid solution dropwise at a rate of 2mL / min to a sample bottle containing 0.5g NCM622 positive electrode powder Among them, the solid-to-liquid ratio is 50g / L. In addition, the reaction temperature is controlled by an oil bath to be 60°C, the rotational speed is controlled to be 600rpm by magnetic stirring, and the reaction time is controlled to be 60min. The volume concentration of peracetic acid in the peracetic acid solution is The leaching rate of lithium, nickel, cobalt, and manganese is 100%, 100%, 100%, and 100% through ICP-MS testing of the content of lithium, nickel, cobalt, and manganese elements in the leaching solution.
[0058] Obtain nickel-containing material and manganese-cobalt-lithium leachate through dimethylglyoxime, adjust the pH=7.8 of the leachate with sodium hydroxide or a...
Embodiment 3
[0060] Discharge the waste 622-type nickel-cobalt-manganese ternary lithium-ion battery and disassemble it in a glove box to obtain NCM622 positive electrode powder; add 10mL peracetic acid solution dropwise at a rate of 2mL / min to a sample bottle containing 0.5g NCM622 positive electrode powder Among them, the reaction temperature is controlled by an oil bath to be 60°C, and the reaction time is 60min. In addition, the rotating speed is controlled to be 600rpm by magnetic stirring, the solid-liquid ratio is controlled to be 50g / L, and the volume concentration of peracetic acid in the peracetic acid solution is The content of lithium, nickel, cobalt and manganese elements in the leaching solution was tested by ICP-MS, and the leaching rates of lithium, nickel, cobalt and manganese were 99.99%, 99.97%, 99.98% and 99.99%, respectively.
[0061] Use Cyanex 272 extractant, PC-88A extractant, and D2EHPA extractant for multiphase extraction of cobalt, nickel, and manganese. The extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com