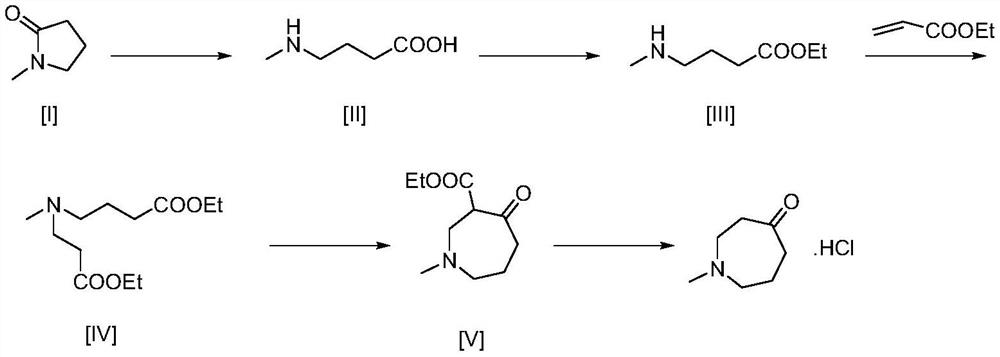
Synthesis method and application of 1-methylhexahydroazepine-4-one hydrochloride
A technology for the synthesis of methylhexahydronitrogen and its synthesis method, which is applied in the field of synthesis of azelastine hydrochloride pharmaceutical intermediates, can solve the problems of cumbersome operation of the overall reaction route, unfavorable industrial production, and long production cycle of the process, and achieve green environmental protection industrialization, The effect of low cost and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of 1-methyl-4-(nitromethyl) piperidin-4-ol
[0034] Nitromethane (66 mL, 1.22 mol) and MeONa (2.21 g, 0.041 mol) were added to a solution of compound 1-methylpiperidin-4-one (100 mL, 0.81 mol) in EtOH (100 mL). After 30 min, additional ethanol (150 mL) was added to facilitate stirring. The reaction mixture was stirred at room temperature for 48 h, then filtered. The separated solid was washed with MTBE to give the product 1-methyl-4-(nitromethyl)piperidin-4-ol (114 g, yield 80.4%).
[0035] H-NMR (d-DMSO, 400MHz): δ5.01(s, 1H), 4.47(s, 2H), 2.41-2.40 (m, 2H), 2.21-2.15(m, 2H), 2.12(s, 3H) ), 1.64-1.53 (m, 4H).
Embodiment 2
[0036] Embodiment 2: Preparation of 4-(aminomethyl)-1-methylpiperidin-4-ol
[0037] A mixture of 1-methyl-4-(nitromethyl)piperidin-4-ol (6 g, 35 mmol) and Raney nickel 600 mg in methanol 250 ml was stirred at room temperature under hydrogen atmosphere for 20 h. The mixture was filtered through celite and evaporated under reduced pressure. The residue was obtained without further purification as 4-(aminomethyl)-1-methylpiperidin-4-ol (4 g, 74%).
Embodiment 3
[0038] Embodiment 3: the preparation of N-methylhexahydroazepin-4-one hydrochloride
[0039] Dissolve 5g (34.67mmol) of 4-(aminomethyl)-1-methylpiperidin-4-ol in 50ml of glacial acetic acid, cool down to 0°C, slowly add sodium nitrite (2.46g, 35.71mmol) in water (25ml) solution, keep warm at 0°C, stir and react overnight, the reaction is complete, add 250ml DCM and adjust the pH of the reaction solution to 7-8 with sodium bicarbonate, separate the DCM layer, wash the water layer twice with 50ml DCM, combine the organic layer and concentrate , add 20ml of isopropanol to dissolve the oil, adjust the pH value to <6 with 3ml of hydrogen chloride isopropanol solution, cool down and crystallize to obtain N-methylhexahydroazepin-4-one hydrochloride, dry under reduced pressure and vacuum , to obtain a solid (4.96 g, 87.0%).
[0040] H-NMR (d-DMSO, 400MHz): δ11.69(s, 1H), 3.56-3.48(m, 2H), 3.46-3.43(m, 2H), 3.44(M, 2H), 2.79(s, 3H ), 2.69-2.50 (m, 2H), 2.07-1.99 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com