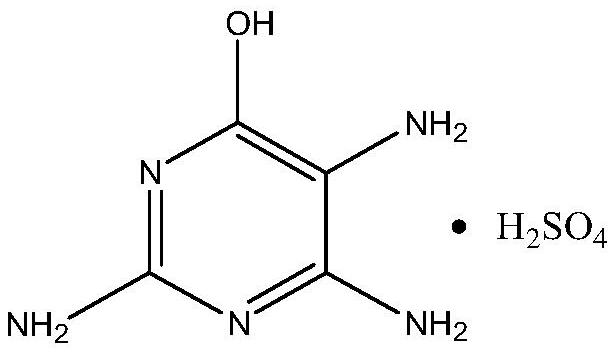
Preparation method of 6-hydroxy-2, 4, 5-triaminopyrimidine sulfate
A technology of triaminopyrimidine and sulfate, applied in the fields of medicine and chemical industry, can solve the problems of easily causing product quality risks, poor stability of guanidine hydrochloride, and increasing production costs, and achieves the effect of avoiding reaction safety risks and being beneficial to sustainable development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) 40 g of guanidine sulfate was added to 100 g of methanol, then 41 g of solid sodium methoxide was added, the temperature was raised to 60° C. with stirring, and the mixture was freed for 1 hour. Then, 37 g of methyl cyanoacetate was slowly added dropwise to the reaction solution, and the cyclization reaction was completed by refluxing for 3 hours to obtain a cyclization reaction solution.
[0038] (2) after the step (1) cyclization reaction solution is distilled to recover the solvent methanol, add 150 g of water and stir, be warming up to 80 ° C after dissolving, then be cooled to 20 ° C, dropwise the mass percentage concentration of 50% sulfuric acid 90g acidification , adjust pH to 1, set aside.
[0039] (3) 27g sodium nitrite is dissolved in the water of 40.5g, is mixed with the sodium nitrite solution that mass percent concentration is 40%, is slowly added dropwise in step (2) and reacts, the reaction temperature is 20 ℃, and the reaction end point is The nitr...
Embodiment 2
[0043] (1) 40 g of guanidine sulfate was added to 100 g of methanol, then 51.5 g of solid sodium ethoxide was added, the temperature was raised to 60° C. with stirring, and freed for 1 hour. Then, 36 g of methyl cyanoacetate was slowly added dropwise to the reaction solution, and the reflux reaction was completed for 3 hours to obtain a cyclization reaction solution.
[0044] (2) after the step (1) cyclization reaction solution is distilled to recover the solvent methanol, add 150 g of water and stir, be warming up to 80 ° C and dissolve the clear, then be cooled to 20 ° C, drop the mass percentage concentration of 90 g of sulfuric acid that is 50% Acidify, adjust pH to 1, and set aside.
[0045] (3) 27g sodium nitrite is dissolved in the water of 40.5g, is mixed with the sodium nitrite solution that the mass percent concentration is 40%, is slowly added dropwise in step (2) and reacts, the reaction temperature is 10 ℃, and the reaction end point is The nitrous solution turns...
Embodiment 3
[0049] (1) 40 g of guanidine sulfate was added to 100 g of methanol, then 73 g of solid sodium tert-butoxide was added, the temperature was raised to 60° C. with stirring, and freed for 1 hour. Then, 36 g of methyl cyanoacetate was slowly added dropwise to the reaction solution, and the reflux reaction was completed for 3 hours to obtain a cyclization reaction solution.
[0050] (2) after the step (1) cyclization reaction solution is distilled to recover the solvent methanol, add 150 g of water and stir, be warming up to 80 ° C after dissolving clear, then be cooled to 10 ° C, dropwise the mass percentage concentration of 50% sulfuric acid 90g acidification , adjust pH to 2, set aside.
[0051] (3) 27g sodium nitrite is dissolved in the water of 40.5g, is mixed with the sodium nitrite solution that mass percent concentration is 40%, is slowly added dropwise in step (2) and reacts, the reaction temperature is 20 ℃, and the reaction end point is The nitrous solution turns the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com