Method for removing impurity compounds in lidocaine and obtained product
A lidocaine and compound technology, applied in the field of drug detection and impurity control, can solve the problems affecting the sustainable and healthy development of the pharmaceutical industry, the quality assurance of drug products, and the discovery of unknown impurities that affect the safety of public medication, and achieve strong specificity, Ease of operation and control, and the effect of ensuring drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] 1. Preparation of chloroacetyl-2,6-dimethylaniline
[0098]
[0099] Include the following steps:
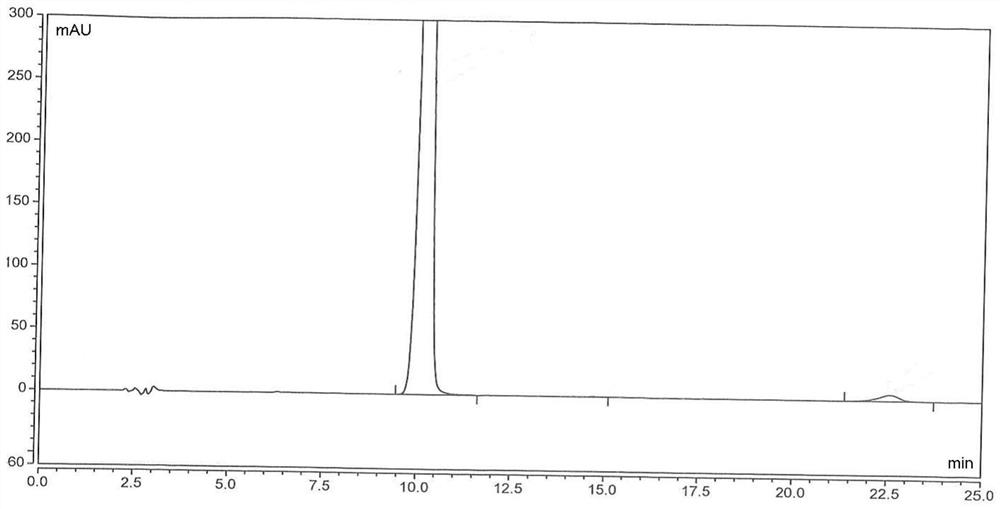
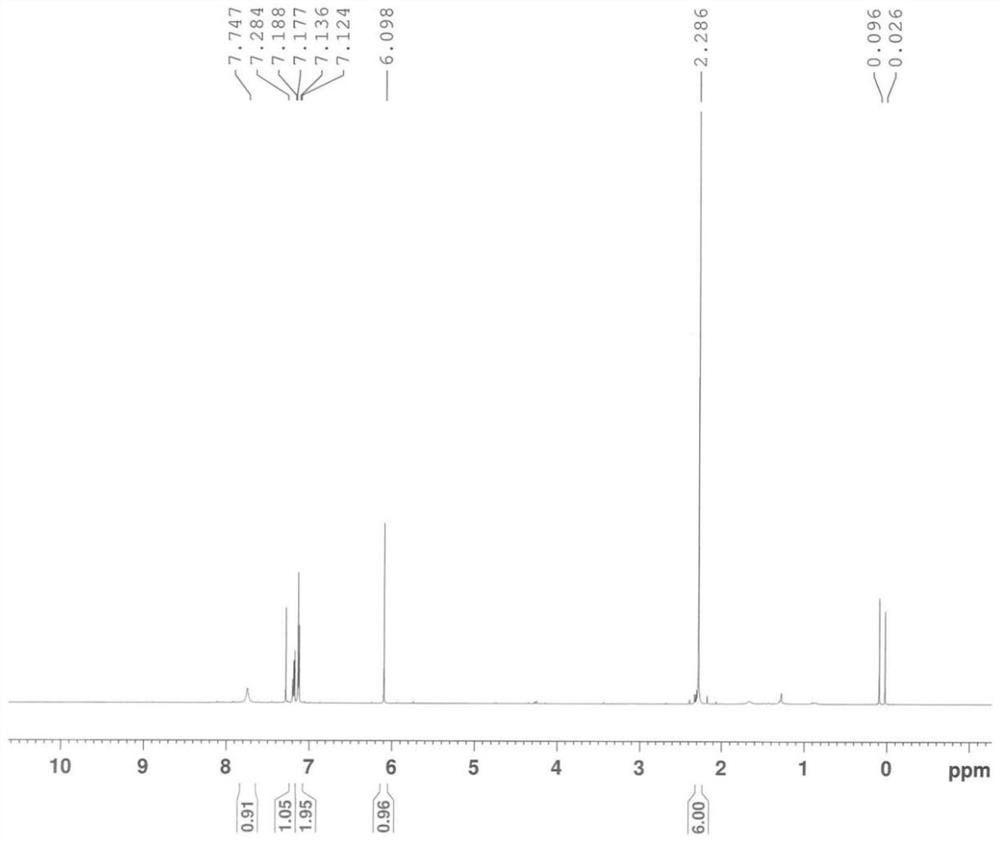
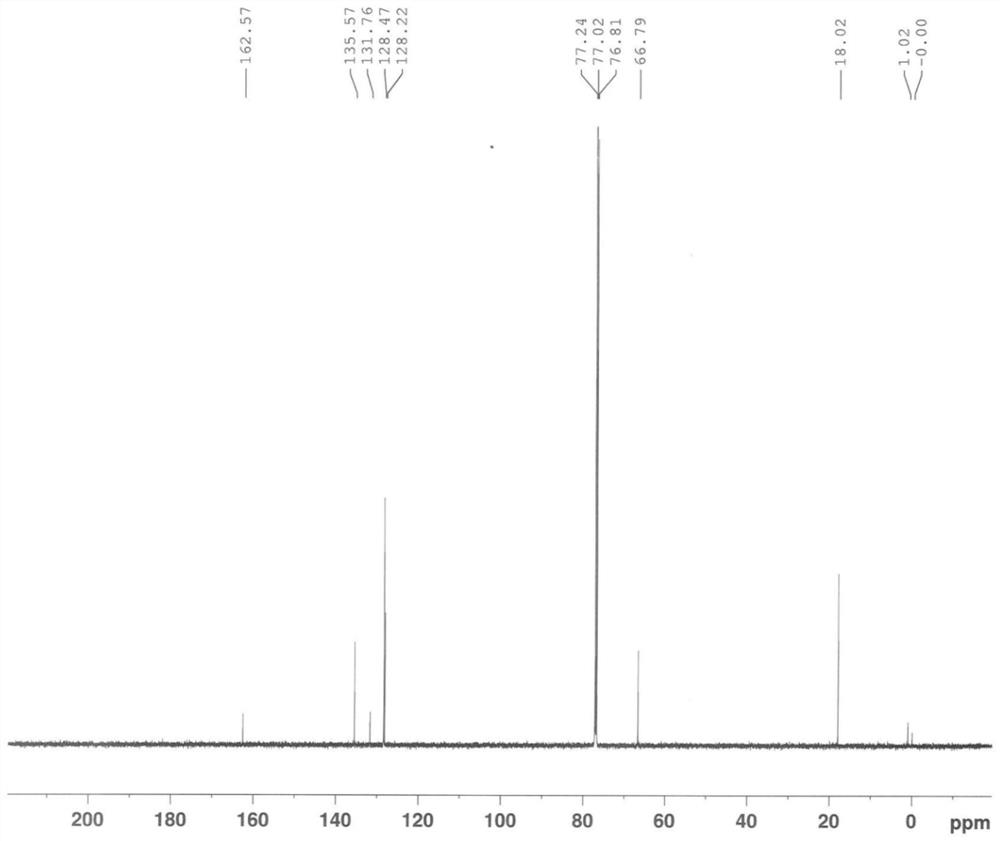
[0100] A, inert gas (nitrogen) protection, add 16kg (about 132mol) 2,6-dimethylaniline, 11.09kg (about 132mol) sodium bicarbonate and 96L methylene dichloride in the reactor, stir, add 16.4kg ( About 145mol) chloroacetyl chloride (control the temperature in the kettle -5~15℃), stir the reaction at -5~15℃ for 1h, GC (gas chromatography) or TLC (thin layer chromatography) monitor the reaction is basically complete (2,6- The residual amount of dimethylaniline≤2wt%) ends after;
[0101] b. Add 16kg of purified water to the kettle, stir evenly, then distill under reduced pressure (vacuum degree≤-0.09MPa, temperature 40~45°C) until no distillate is produced, then add 176kg of purified water to the kettle, at 15~35 After stirring at °C for 2 h, centrifugation, and drying by blasting (50-60 °C), chloroacetyl-2,6-dimethylaniline was obtained as a white powdery solid with a yi...
Embodiment 2 and 3
[0160] The same content as Example 1 will not be repeated, the difference is that in the step a of preparing chloroacetyl-2,6-dimethylaniline, the consumption of chloroacetyl chloride is changed to 132mol and 158.4mol respectively, namely: 1 equivalent , 1.2 equivalents, and finally obtain chloroacetyl-2,6-dimethylaniline, white powdery solid, the yield is all ≥90% (calculated as 2,6-dimethylaniline), the HPLC purity is all ≥98%, impurity The content of A is in the range of 0.6% to 1.5%.
Embodiment 4~6
[0162] The same content as Example 1 is not repeated, the difference is that in the step a of preparing chloroacetyl-2,6-dimethylaniline, the consumption of sodium bicarbonate is changed to 106mol, 158.4mol, 198mol respectively, namely: 0.8 equivalents, 1.2 equivalents, and 1.5 equivalents, and finally chloroacetyl-2,6-dimethylaniline was obtained as a white powdery solid, the yields were all ≥95% (calculated as 2,6-dimethylaniline), and the HPLC purity was uniform. ≥98%, the content of impurity A is in the range of 0.6% to 1.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com