Preparation method of decarboxylase and 5-hydroxytryptamine
A decarboxylase and serotonin technology, applied in the fields of genetic engineering and biocatalysis, can solve the problems of unhealthy bacterial growth, poor expression, and low enzyme activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Molecular Cloning
[0025] Design specific primer pairs to introduce the desired substitution at the corresponding base at the desired mutated amino acid position. Using the extracted genomic DNA of Aspergillus niger ATCC 1015 as a template, an exon fragment encoding the corresponding amino acid sequence of SEQ ID No. 1 (GenBank: EHA26219.1) was amplified by PCR and purified.
[0026] Using overlap extension PCR technology, each exon sequence is recombined and connected to form a PCR product containing a complete coding sequence, and the nucleic acid sequence of the coding region is as described in SEQ ID No.2.
[0027] The purified PCR product was digested with NdeI-HindIII, and then ligated with the pET-30a vector digested at the same site by T4 ligase. The ligation product was transformed into E. coli BL21 (DE3) competent cells, spread on LB agar medium (containing 50 mg / L kanamycin), and single colonies were picked into LB liquid medium (containing 50 mg...
Embodiment 2
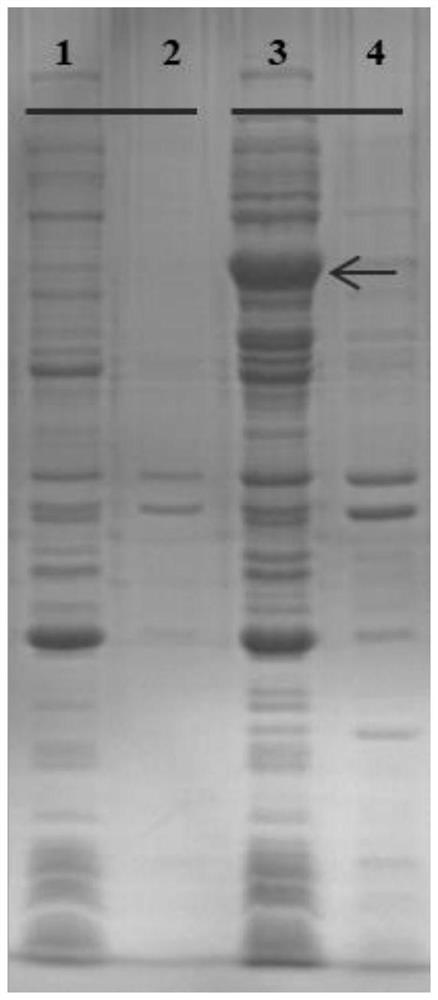
[0028] Example 2: Recombinant soluble expression of unknown protein encoded by SEQ ID No. 1 in E. coli.
[0029] The deposited clones were activated on LB agar medium. Then a single colony was inoculated into LB liquid medium (containing 50 mg / L kanamycin), and incubated at 37°C with shaking for 12 h. Transfer 1 mL of the culture to 50 mL of fresh LB liquid medium (containing 50 mg / L kanamycin), incubate at 37 °C with shaking until the OD600 reaches about 0.6, add IPTG (final concentration 0.4mM) at 25 °C Incubate for 16 h to induce protein expression.
[0030] After incubation, the culture was centrifuged at 4,000 g for 10 min at 4°C, the supernatant was discarded, and the E. coli cells were collected. The collected E. coli cells were resuspended in 15 mL of pre-chilled phosphate buffered saline (PBS) pH 7.0, and the E. coli cells were sonicated at 4°C. The cell disrupted liquid was centrifuged at 6,000 g at 4°C for 15 min to remove the precipitate, and the obtained supern...
Embodiment 3
[0031] Example 3: Decarboxylation experiment of 5-hydroxytryptophan by decarboxylase encoded by SEQ ID No. 1
[0032] L-5-hydroxytryptophan was dissolved in 100 mM phosphate buffered saline (PBS) pH=7.0 so that the final concentration of L-5-hydroxytryptophan in the final reaction system solution was 25 mM. A 10% volume sample of the enzyme reagent was added to the above solution. Continue shaking on a shaker (800 rpm) for 3 hours at 30° C., take a sample and detect the conversion of the substrate by high performance liquid chromatography. In some reactions, pyridoxal phosphate (PLP) solution with a final concentration of 50 μM was also added. After the reaction, the concentrations of the residual substrate 5-HTP and the product 5-HT were measured by HPLC quantitative analysis. The experimental results are shown in Table 1.
[0033] sample name Substrate 5-HTP (mM) Product 5-HT (mM) Molar conversion % enzyme-free solution 25.0 0 0 Empty plasmid e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com