Oxcarbazepine derivative, immunogen, anti-oxcarbazepine specific antibody as well as preparation method and application of oxcarbazepine specific antibody
A technology of oxcarbazepine and its derivatives, which is applied in the preparation method of peptides, biological testing, immunoglobulin, etc., can solve the problems of cumbersome operation, inconvenient large-scale clinical testing, and difficult control of the process, and achieve high sensitivity and effective Conducive to clinical promotion and use, high coupling efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
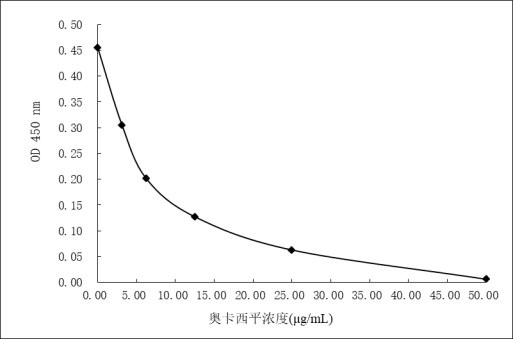
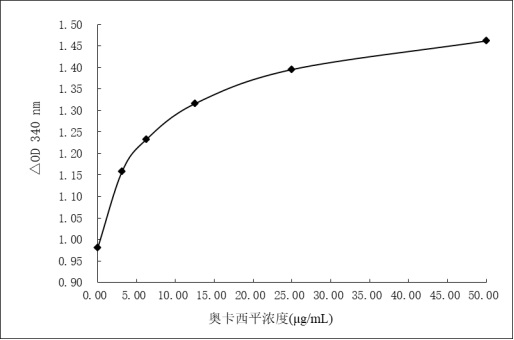
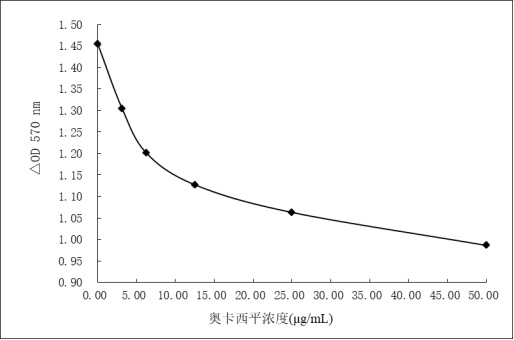
Image
Examples
Embodiment 1
[0096] Example 1: Synthesis of Oxcarbazepine Derivatives
[0097] Oxcarbazepine derivatives were synthesized by the following synthetic routes:
[0098]
[0099] The specific synthesis steps are as follows:
[0100] (1) Synthesis of compound 2:
[0101]
[0102] Weigh 2.5 g of potassium nitrosodisulfonate (Fremy's salt, (KSO 3 ) 2 NO), 9.32 mmol) and 1.8 g Na 2 HPO 4 (12.7 mmol), put it into a beaker, add 95 ml of double distilled water to dissolve, adjust the pH to 7.22; weigh 0.6 g of compound 1 (12.76 mmol) into 60 ml of acetone solution; mix the above two solutions and stir vigorously to obtain a purple color solution; the above purple solution was added to the acetone solution, stirred continuously for 10 minutes, filtered, and placed in a refrigerator overnight; the overnight solution was concentrated by an argon rotary evaporator, extracted with 500 ml of ether, and after the extraction was completed, the organic The phase solvent was evaporated; the evapora...
Embodiment 2
[0121] Example 2: Preparation of Oxcarbazepine Immunogen
[0122] The specific steps of the preparation method of oxcarbazepine immunogen are as follows:
[0123] (1) Preparation of carrier protein solution: Dissolve recombinant human serum albumin in 0.35mol / L potassium phosphate buffer (pH=8.5), and the final concentration of recombinant human serum albumin is 5.0mg / mL to obtain carrier protein solution;
[0124] (2) Preparation of oxcarbazepine derivative solution: 250.0 mg of the above oxcarbazepine derivative, 7.5 mL of dimethylformamide, 7.5 mL of ethanol, and 15.0 mL of potassium phosphate buffer (10.0 mmol / L, pH= 8.0), 150.0 mg of 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide, and 90.0 mg of N-hydroxysulfosuccinimide were mixed, stirred and dissolved for 3 hours to obtain oxcarbazepine Derivative solution;
[0125] (3) Synthesis of oxcarbazepine immunogen: The oxcarbazepine derivative solution obtained in step (2) was added dropwise to the carrier protein solution...
Embodiment 3
[0126] Example 3: Preparation of anti-oxcarbazepine-specific antibodies
[0127] The specific steps of the preparation method of anti-oxcarbazepine-specific antibody are as follows:
[0128] (1) Dilute the above oxcarbazepine immunogen with 0.15mol / L sodium phosphate buffer (pH=7.0) to a final concentration of 3.5mg / mL to obtain an artificial antigen solution, and then mix 3.0mL of the artificial antigen solution with an equal amount of Freund's complete adjuvant was mixed and injected into experimental animals rabbits at multiple points;
[0129] (2) After 4 weeks, 3.0 mL of the same artificial antigen solution and the same amount of incomplete Freund's adjuvant were injected into the above-mentioned experimental animal rabbits at multiple points, and then once every 5 weeks, for a total of 6 injections;
[0130] (3) Blood is collected from the experimental animal rabbit that has been injected in step (2), and is separated and purified to obtain an anti-oxcarbazepine-specifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com