Application of recombinant human hyaluronidase preparation
A technology of human hyaluronidase and hyaluronic acid, which is applied in the application field of recombinant human hyaluronidase preparations, can solve the problems of ineffective treatment, low risk of allergy, poor curative effect, etc., and achieves high concentration of active protein and effective treatment. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] Example 1 Preparation of human hyaluronidase protein
[0237] The CHO cells were cultured in suspension in the self-developed serum-free medium, and the self-developed serum-free feed medium was used for fed-feed controlled culture, which was gradually expanded to a 30L reactor scale through shake flask culture.
[0238] When cultured for 3-4 days, the daily amount of feed medium added to the bioreactor is 2%-5% of the actual culture volume in the bioreactor. The culture temperature was controlled at 35°C to 37°C by adding 10% Na 2 CO 3 and CO 2 The pH is controlled at 7.0; the ventilation volume of the reactor is controlled at 0.015-0.15vvm; the rotational speed is controlled at 80-150rpm; the dissolved oxygen value is controlled at 20%-40%. During cell culture, samples were taken every day to monitor temperature, pH, glucose concentration, lactate concentration, osmolality and protein expression; when the CHO cell viability was lower than 80% or the culture period ...
Embodiment 2
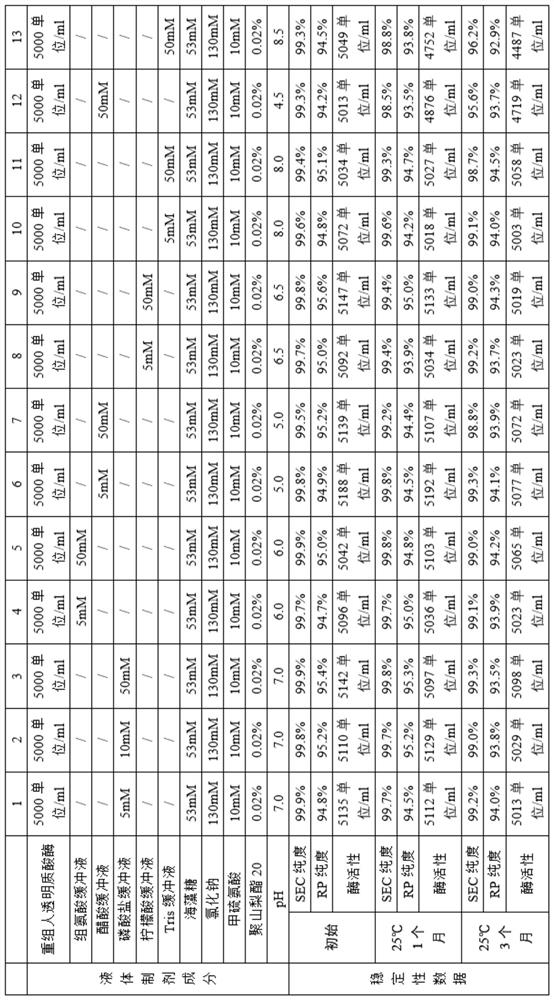
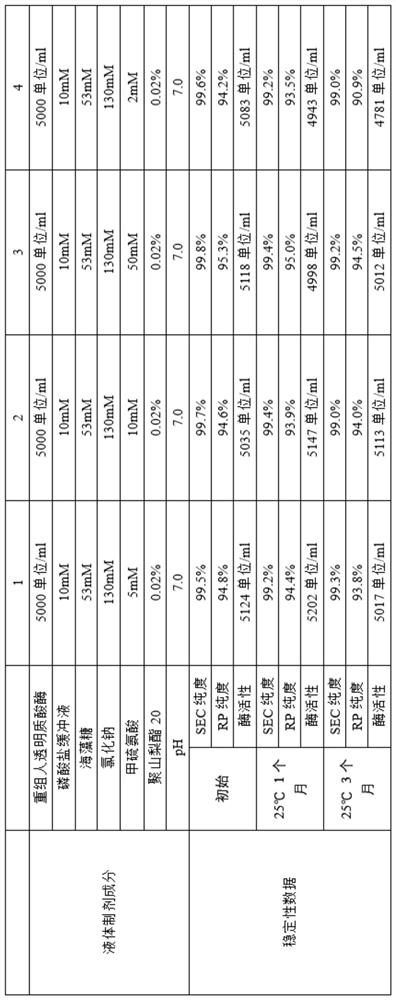
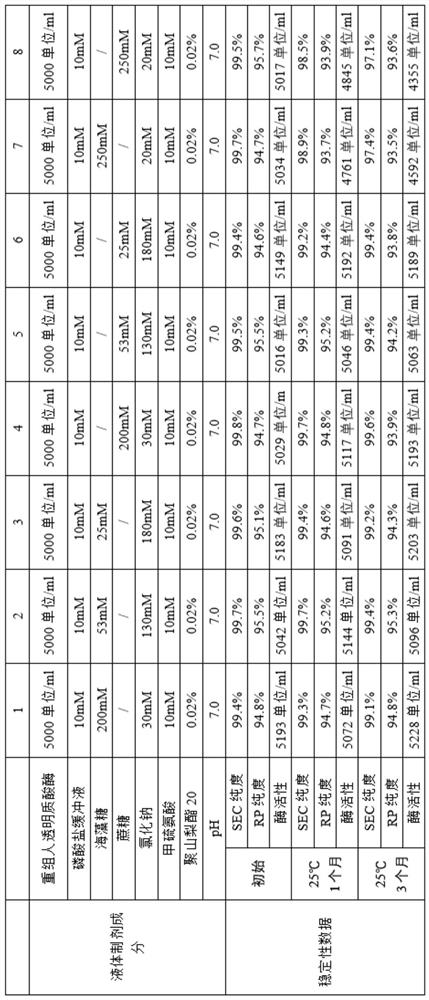
[0240] Example 2 Research on the liquid preparation of recombinant human hyaluronidase
[0241]The recombinant human hyaluronidase stock solution obtained in Example 1 was exchanged into liquid preparations with different compositions, and the concentration of the recombinant human hyaluronidase was adjusted to the expected concentration. All liquid formulations were sterile filtered through 0.22 μm low protein adsorption filters and filled under sterile conditions into sterile 5 ml glass vials, stoppered with fluororesin layered butyl rubber stoppers and aluminum / plastic lift-off type (flip-off) Seal and cap. The fill volume is 2ml. These formulations were stored at different temperatures and samples were taken at specified time intervals to investigate formulation stability, and the stability data for different liquid formulations were summarized. The samples were set out at 25°C for accelerated experiments, and samples were taken at different times to analyze the protein ...
Embodiment 3
[0248] Example 3 Study on the freeze-dried preparation of recombinant human hyaluronidase
[0249] Different recombinant human hyaluronidase liquid preparations were prepared first, and then freeze-dried according to Table 1, and the stability of different freeze-dried preparations was studied after freeze-drying.
[0250] Table 1
[0251]
[0252]
[0253] like Figure 7 Buffers indicated were 5 mM and 50 mM phosphate buffer, histidine buffer, acetate buffer, citrate buffer and Tris buffer, pH 5.0, 6.0, 6.5, 7.0, 8.0 conditions provided The lyophilized preparation of recombinant human hyaluronidase has better stability.
[0254] like Figure 8 As shown, the lyophilized formulations containing recombinant human hyaluronidase in the range of 5, 10 and 50 mM methionine were more stable.
[0255] like Figure 9 As shown, the lyophilized preparation of recombinant human hyaluronidase containing the protective agent sodium chloride concentration of 50-170 mM, trehalose 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com