Flutriafol hapten, flutriafol antigen, flutriafol antibody, flutriafol detection device and preparation and application thereof
A technology of triadol and hapten, which is applied in the field of immunological detection of food safety, can solve the problems of high residue, complex process and tediousness, etc., and achieve the effect of simple and effective synthesis steps, simple operation process and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
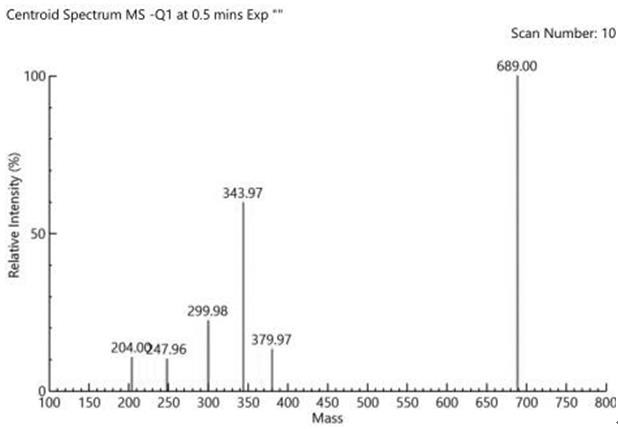
Image
Examples
Embodiment 1
[0034] In this example, dimethyl sulfide is selected from 99% dimethyl sulfide supplied by Sinopharm Chemical Reagent Co., Ltd., concentrated sulfuric acid is selected from 95%~98% sulfuric acid supplied by Sinopharm Chemical Reagent Co., Ltd., and methanol is selected from Sinopharm Chemical Co., Ltd. Analytical pure methanol supplied by Reagent Co., Ltd., tert-butanol selected from Sinopharm Chemical Reagent Co., Ltd., analytically pure tert-butanol, potassium hydroxide, analytically pure potassium hydroxide supplied by Sinopharm Chemical Reagent Co., Ltd., and anhydrous sodium sulfate selected 99% anhydrous sodium sulfate supplied by Sinopharm Chemical Reagent Co., Ltd., 60% sodium hydride supplied by Sinopharm Chemical Reagent Co., Ltd., 60% sodium hydride supplied by Sinopharm Chemical Reagent Co., Ltd., and analytical pure sodium hydroxide supplied by Sinopharm Chemical Reagent Co., Ltd. Sodium hydroxide, the following examples 2-10 all use the reagents in this example 1....
Embodiment 2
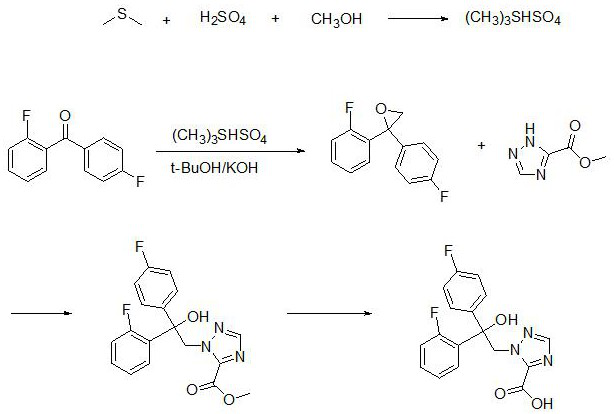
[0035] Example 2 Synthesis and identification of fenconazole hapten
[0036] The preparation method of fenconazole hapten, comprises the following steps:
[0037] S1. Add dimethyl sulfide to the reaction vessel, and slowly add concentrated sulfuric acid and methanol dropwise at 5°C. After 4.5 hours of reaction at room temperature, a first reaction solution is obtained, in which the mixture of dimethyl sulfide, concentrated sulfuric acid and methanol is obtained. The molar ratio is 1:(0.5-1.5):(0.2-1);
[0038] S2. in the first reaction solution, add 2,4-difluorobenzophenone, then add tert-butanol to help dissolve, add potassium hydroxide particles in batches, and the mol ratio of 2,4-difluorobenzophenone and potassium hydroxide is 1: (5-10), react overnight at 45°C to obtain the second reaction solution, pour the second reaction solution into purified water, add ethyl acetate for extraction 3 times, wash with saturated brine 2 times, anhydrous After drying with sodium sulfat...
Embodiment 3
[0044] Example 3 Preparation and identification of antigens for immunization and coating of fenconazole
[0045] 3.1 Preparation of Antigen for Fenconazole immunization: Weigh 30mg of Fenconazole hapten prepared in Example 2, dissolve it in 2ml of N,N-dimethylformamide (DMF), add 18mg of N-hydroxybutanediol imide (NHS), 26 mg 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC.HCl), react at room temperature for 6 h to prepare an activation solution; 60 mg bovine serum albumin ( BSA) was dissolved in 2ml of 0.05M boric acid buffer at pH 9.0, added 1ml of DMF, 0.5ml of the above activation solution, reacted at room temperature for 4h, and then dialyzed with PBS (0.02 mol / L, pH=7.4 phosphate buffer), every 4 hours h Change the medium once, change the medium 7-8 times, centrifuge at 4000 rpm for 5 min after dialysis, and take the supernatant to obtain the falconazole hapten-BSA conjugate, which is the antigen for falconol immunization, which is then packaged in aliquo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com