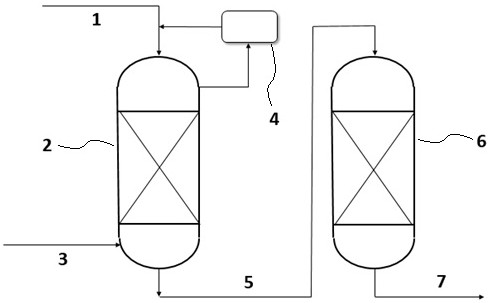
Method for synthesizing lactide from L-lactic acid by adopting fixed bed reactor
A fixed-bed reactor and lactide technology, applied in the direction of organic chemistry, can solve problems such as easy coking, unfavorable industrial production, long process flow, etc., achieve high-temperature resistant catalytic performance, easy industrial implementation, and reduce free acid content Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of silicon-substituted aluminum phosphate molecular sieve catalyst: mix phosphoric acid solution with deionized water, then add inorganic aluminum salt, white carbon black and tetraethylammonium hydroxide to the mixed solution in turn, stir evenly; heat the above mixed solution at 55°C Heating in a water bath for 4 hours while continuing to stir, the obtained gel was poured into a polytetrafluoroethylene-lined reaction kettle, and then put into a dynamic crystallization box for crystallization at a heating rate of 4.5 °C / h, and heated to 220 °C. ℃ constant temperature reaction for 50h, cooling, washing, drying and roasting after crystallization is completed to obtain silicon-substituted aluminum phosphate molecular sieve. Si in the catalyst is SiO 2 meter, Al to Al 2 O3 Count, P to P 2 O 5 Gauge, SiO 2 :Al 2 O 3 1:2, SiO 2 :P 2 O 5 is 1:2, and the average particle size of the catalyst is 0.5 μm.
[0028] The liquid hourly volume space velocity of L...
Embodiment 2
[0032] Preparation of silicon-substituted aluminum phosphate molecular sieve catalyst: mix phosphoric acid solution with deionized water, then add inorganic aluminum salt, white carbon black and tetraethylammonium hydroxide to the mixed solution in turn, stir evenly; heat the above mixed solution at 55°C Heating in a water bath for 5 hours, while continuing to stir, the obtained gel was poured into a PTFE-lined reaction kettle, and then put into a dynamic crystallization box for crystallization at a heating rate of 5 °C / h, and heated to 220 °C. ℃ constant temperature reaction for 55h, after crystallization is completed, cooling, washing, drying and roasting, to obtain silicon-substituted aluminum phosphate molecular sieve. Si in the catalyst is SiO 2 meter, Al to Al 2 O 3 Count, P to P 2 O 5 Gauge, SiO 2 :Al 2 O 3 1:2, SiO 2 :P 2 O 5 is 1:2.4, and the average particle size of the catalyst is 0.6 μm.
[0033] The liquid hourly volume space velocity of L-lactic acid r...
Embodiment 3
[0037] Preparation of silicon-substituted aluminum phosphate molecular sieve catalyst: mix phosphoric acid solution with deionized water, then add inorganic aluminum salt, white carbon black and tetraethylammonium hydroxide to the mixed solution in turn, stir evenly; heat the above mixed solution at 50°C Heating in a water bath for 4 hours while continuing to stir, the obtained gel was poured into a polytetrafluoroethylene-lined reaction kettle, and then put into a dynamic crystallization box for crystallization at a heating rate of 4.5 °C / h, and heated to 210 °C. ℃ constant temperature reaction 50h, after crystallization is completed, cooling, washing, drying and roasting, to obtain silicon-substituted aluminum phosphate molecular sieve. Si in the catalyst is SiO 2 meter, Al to Al 2 O 3 Count, P to P 2 O 5 Gauge, SiO 2 :Al 2 O 3 1:2.5, SiO 2 :P 2 O 5 is 1:2, and the average particle size of the catalyst is 0.7 μm.
[0038] The liquid hourly volume space velocity of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com