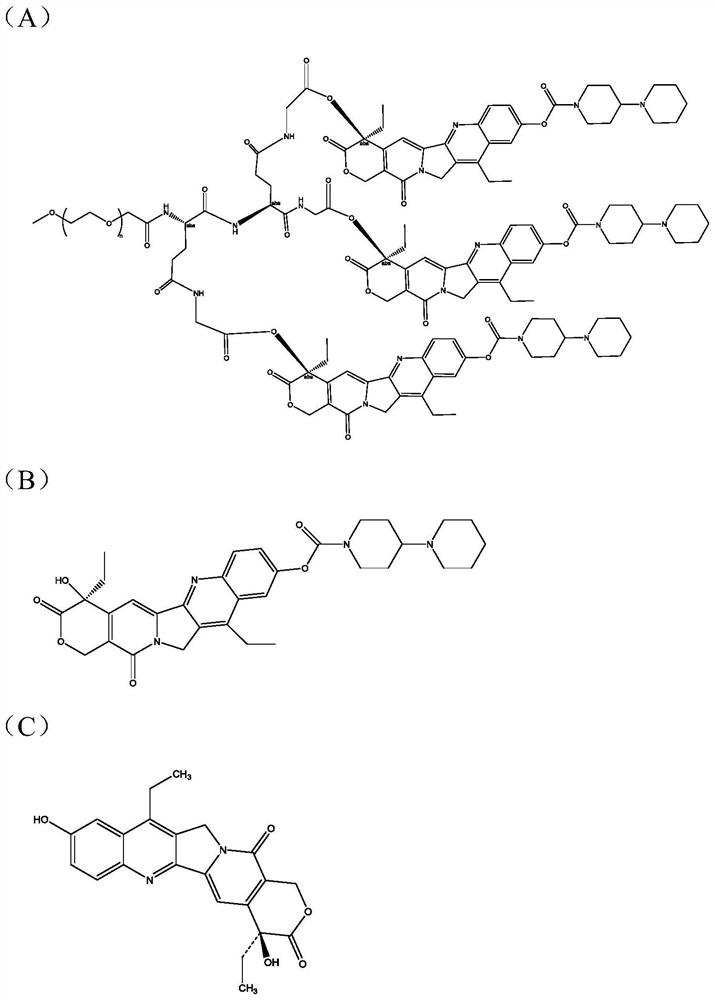
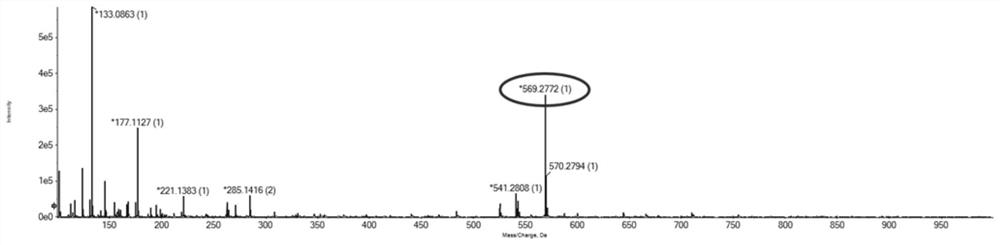
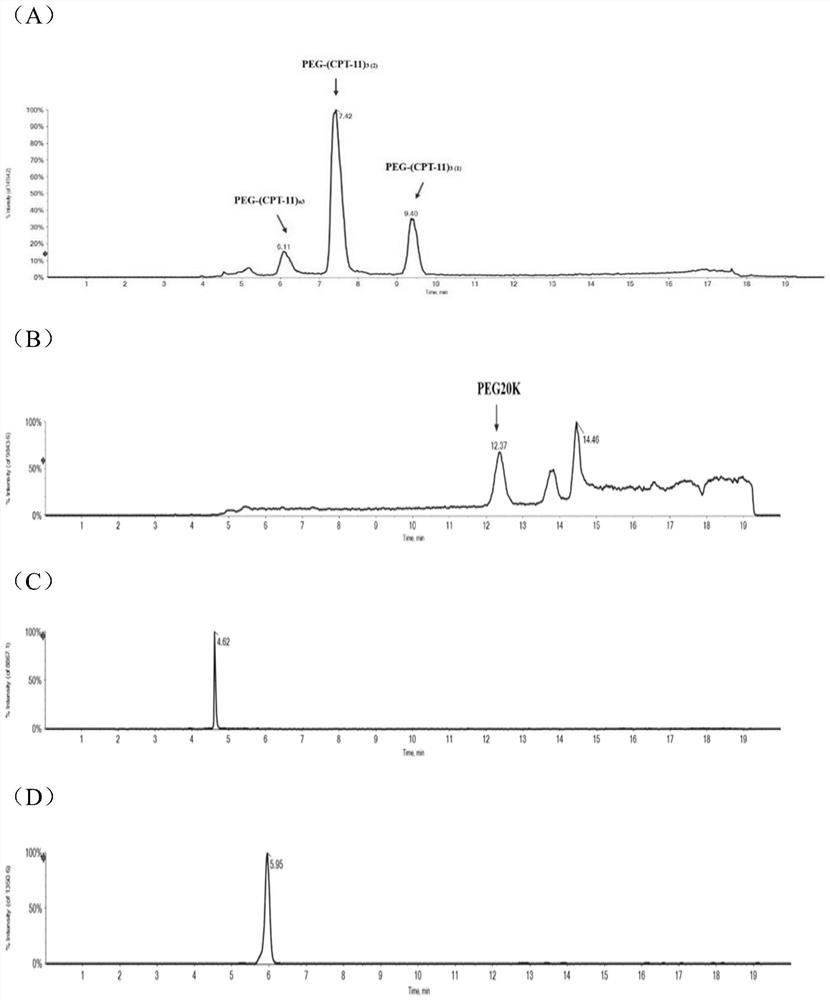
Quantitative analysis method for multivalent PEGylated irinotecan prodrug and metabolite thereof in biological sample
A technology for irinotecan and biological samples, applied in the field of simultaneous quantitative analysis of biological mass spectrometry, can solve the problems of small differences in chromatographic retention behavior, dynamic drug release rules, and complex pharmacokinetic processes, achieving high resolution and good selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In this example, the trivalent PEGylated irinotecan prodrug and its metabolites with different forms in vivo were specifically determined in rat plasma.
[0040] Precision Weighing PEG-(CPT-11) 3 The standard product is about 500mg. After the purity is converted, it is dissolved with sodium chloride solution for injection and diluted to 50mg / mL. The rat is administered 120mg / kg through the tail vein of a single time. The blood collection time points are as follows: 5min, 10min, 30min , 1h, 2h, 4h, 6h, 8h, 10h, 12h, 24h, 36h, 48h. Determination of PEG-(CPT-11) in plasma after tail vein administration in rats 3 and the content of metabolites in different forms, see the plasma drug concentration time curve. Figure 4 .
[0041] Determination of PEG-(CPT-11) 3 The main steps are as follows:
[0042] A 1. Pretreatment of plasma samples:
[0043] 1) Add 50 μL of biological sample to the polyethylene tube (add anticoagulant when collecting plasma samples),
[0044] 2) A...
Embodiment 2
[0075] In this example, the trivalent PEGylated irinotecan prodrug and its metabolites in different forms in vivo were specifically determined in rat urine.
[0076] Rats were given a single tail vein administration of 120mg / kg, collected at 4h, 12h, 24h, 36h, 48h, 60h, 72h, 96h, 120h, 144h, 168h, 192h, 216h, 240h, 264h, 288h, 312h, 336h rat urine. Determination of PEG-(CPT-11) in rat urine 3 and the contents of different forms of metabolites in the body, and the cumulative excretion rate of each form component in rat urine was calculated. The cumulative excretion rate-time curve of rat urine is shown in Figure 5 .
[0077] The main steps are as follows:
[0078] A. Pretreatment of urine samples:
[0079] 1) Add 50 μL of biological sample to a polyethylene tube,
[0080] 2) Add 50 μL of internal standard, vortex to mix,
[0081] 3) Precipitate protein: add 250 μL of acetonitrile, vortex to mix,
[0082] 4) Centrifuge at 13000rpm for 10 minutes,
[0083] 5) Transfer 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com