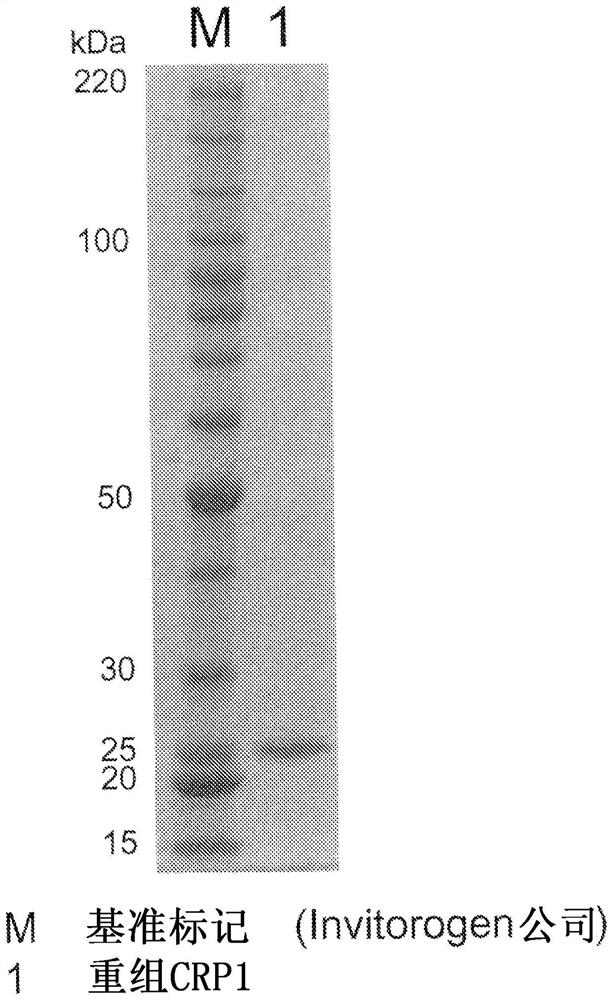
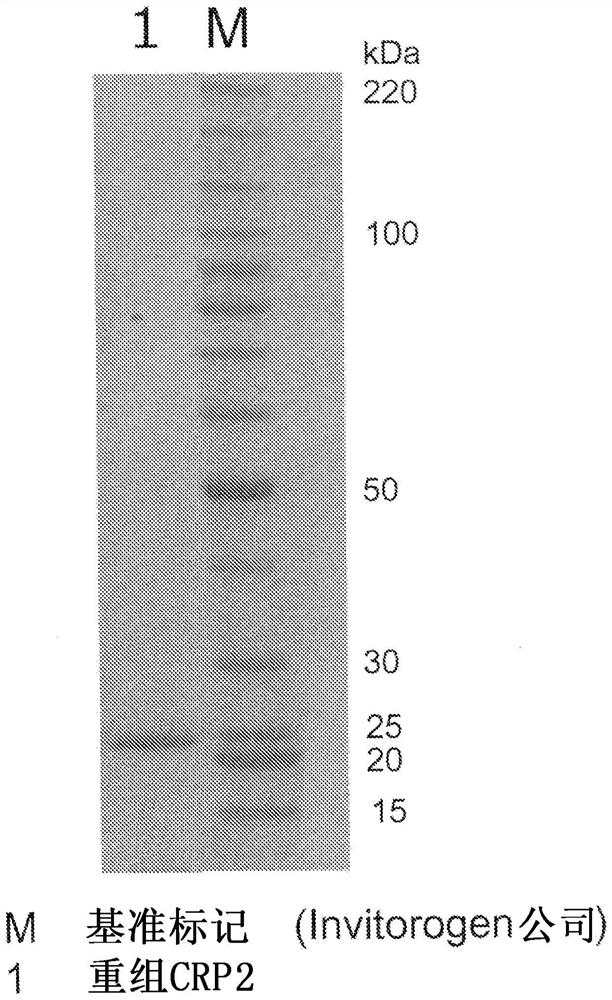
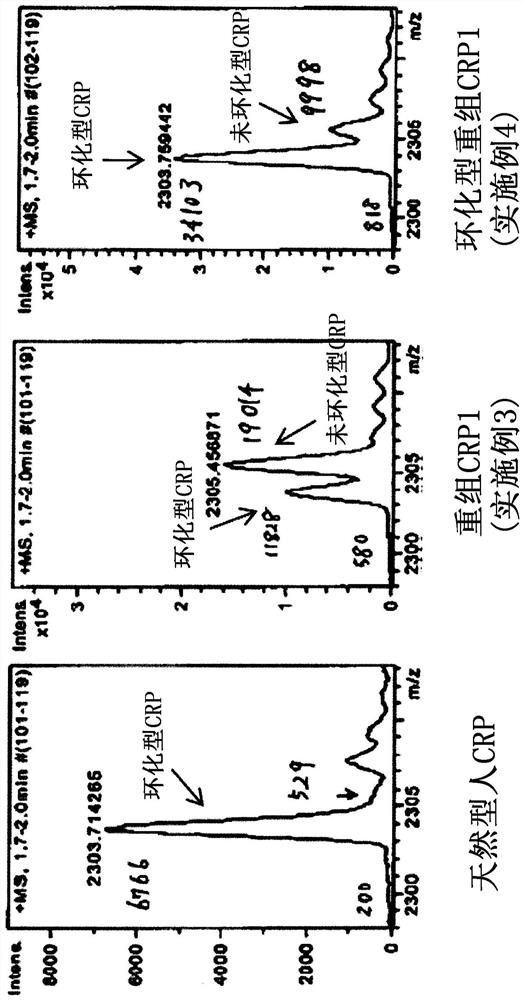
Recombinant C-reactive proteins
A reactive protein and gene recombination technology, applied in the field of C-reactive protein, can solve problems such as non-reporting, and achieve the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1 Introduction of mutations and acquisition of transformants
[0130] (1) Introduction of mutation
[0131]Using the artificially synthesized gene of SEQ ID NO: 7 or SEQ ID NO: 8, which is connected with the alkaline phosphatase secretion signal sequence from Escherichia coli (ATGAAACAAAGCACTATTGCACTGGCACTCTTACCGTTACTGTTTACCCCTGTGACAAAAGCC) and the mature human CRP sequence of SEQ ID NO: 5 or SEQ ID NO: 6 as a template, the sequence Primers No. 9 and 10 amplify the CRP gene. SEQ ID NO: 9 is a forward primer, and SEQ ID NO: 10 is a reverse primer. Restriction enzyme site NdeI or restriction enzyme site BamHI was added to this primer, respectively. A plasmid was constructed by adding the amplified gene fragment and incubating with a vector plasmid pBluescript KSN(+) cleaved with restriction enzymes NdeI and BamHI, and In-Fusion Reaction Mix (manufactured by Takara Bio). In this way, the recombinant plasmid pBKSN_CRP1 containing SEQ ID NO: 7 and the recombinant ...
Embodiment 2
[0134] Example 2 Expression of CRP gene in Escherichia coli
[0135] The colony of the transformant Escherichia coli JM109 (pBKSN_CRP1) obtained in Example 1 was inoculated into 5 mL of LB liquid medium (containing 1.0% glucose and 100 μg / mL of ampicillin) sterilized in a test tube, and cultured at 37° C. for 16 hours. The obtained culture solution was used as a seed culture solution to inoculate 500 mL of LB liquid medium (containing glycerol 0.5%, calcium chloride 0.05%, IPTG 1 mM, and ampicillin 50 μg / mL) into ten 2-L Sakaguchi flasks, Incubate at 30°C for 24 hours with shaking at 180 rpm. The cells were collected by centrifugation from the end of the culture, suspended in a 20 mM Tris-HCl buffer (0.14 M sodium chloride, 2 mM calcium chloride, pH 7.5), crushed by a French press (manufactured by Niro Soavi), and further centrifuged It was separated, and the supernatant liquid was obtained as crude purification liquid 1. Furthermore, crude purification solution 2 was also o...
Embodiment 3
[0136] Example 3 Purification of recombinant CRP
[0137] The crude purified liquid 1 obtained in Example 2 was subjected to affinity purification using Pierce™ p-Aminophenyl PhosphorylCholine Agarose (manufactured by Thermo SCIENTIFIC). The aforementioned resin equilibrated with the buffer used in Example 2 20 mM Tris-HCl buffer (0.14 M sodium chloride, 2 mM calcium chloride, pH 7.5) was mixed and adsorbed with the crude purification solution. The resin was washed with the aforementioned buffer, Recombinant CRP solution 1 was obtained by elution with 20 mM Tris-HCl buffer (0.14 M sodium chloride, 2 mM EDTA 2 mM, pH 7.5). The solution was further concentrated to remove EDTA by adding water using a hollow fiber membrane, and at the same time replaced with the buffer used in Example 2 20 mM Tris-HCl buffer (0.14 M sodium chloride, 2 mM calcium chloride, pH 7.5), and further centrifuged It was concentrated to an appropriate concentration with an ultrafiltration filter (manufactu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com