Enzyme complex hydrogel prepared based on catalysis of glucose oxidase/amino acid metal complex and preparation method of enzyme complex hydrogel
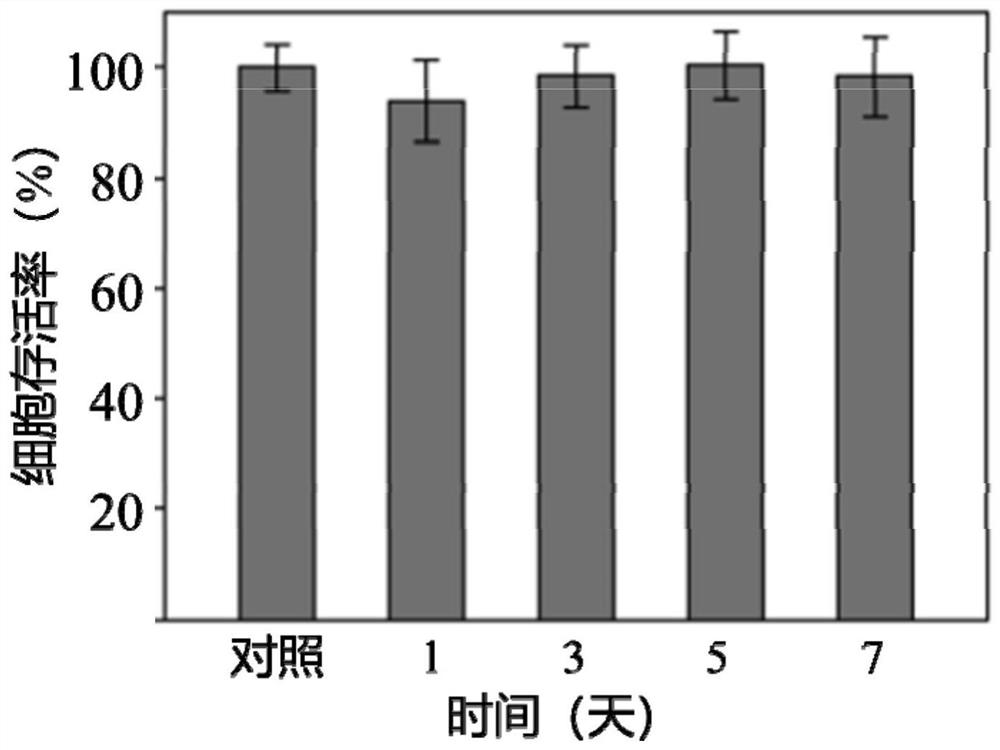
A technology of glucose oxidase and metal complexes, applied in prosthesis, medical science and other directions, can solve problems such as biological toxicity, and achieve the effect of promoting cartilage regeneration, easy operation and good biological safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Polysaccharide-grafted double bond: 2.08 g of chondroitin sulfate was added to 50 mL of deionized water, magnetically stirred for 2 hours to completely dissolve, and HCl (0.1 M) was slowly added dropwise to adjust the pH to 3.5. Then, 675 μL of glycidyl methacrylate (GMA) was added to the solution, and the solution was stirred for 24 hours in a water bath at 50°C. 100 mL of absolute ethanol was added to the reaction system, and a white crude product was precipitated. The crude product was dialyzed against deionized water for three days, and then freeze-dried (-65°C, 1 Pa) to obtain a white flocculent product.
[0035] 2) Prepare the precursor solution: mix 80 mg of polysaccharide grafted with double bonds, 30 mg of N,N-dimethylacrylamide, and then add 100 μL of ferrous glycinate (10 mM) and 50 μL of glucose oxidase (1000 U / mL) after mixing, deionized 800 μL of water was added to the sample bottle, shaken to dissolve it completely and mixed evenly to obtain the precu...
Embodiment 2
[0039] 1) Step is the same as step 1) in Example 1.
[0040] 2) Prepare the precursor solution: 80 mg of grafted double bond polysaccharide, 30 mg of N,N-dimethylacrylamide, and 100 μL of ferrous histidine (10 mM) and 50 μL of glucose oxidase (1000 U / mL) were added in sequence after mixing. 800 μL of deionized water was added to the sample bottle, shaken to dissolve it completely and mixed evenly to obtain the precursor liquid of the hydrogel.
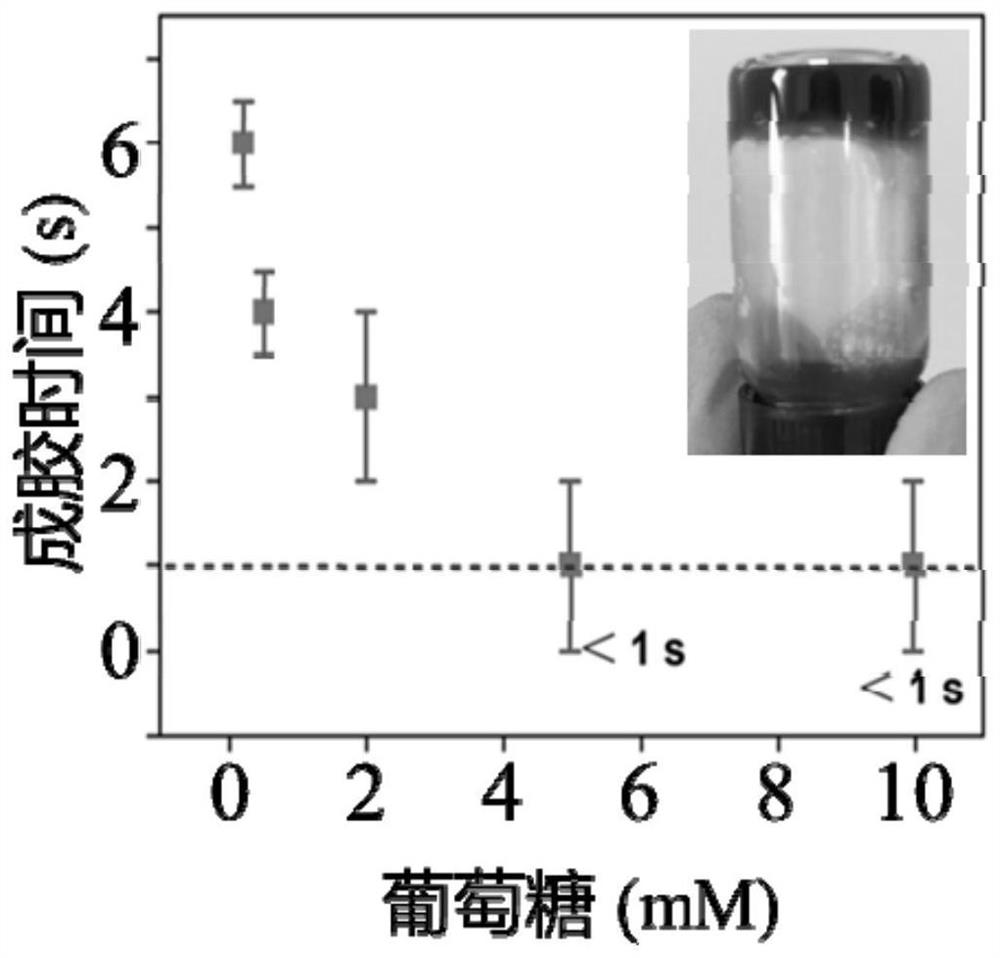
[0041] 3) Preparation of hydrogel: Add 100 μL of glucose (100 mM) solution to the above precursor solution, shake immediately, and mix quickly to obtain a transparent self-supporting hydrogel. The gel formation time About 1s, the compressive modulus is 48.5kPa.
Embodiment 3
[0043] 1) Step is the same as step 1) in Example 1.
[0044] 2) Prepare the precursor solution: mix 80 mg of polysaccharide grafted with double bonds, 30 mg of N,N-dimethylacrylamide, and then add 100 μL of lysine molybdenum (10 mM) and 50 μL of glucose oxidase (1000 U / mL) in sequence to remove 800 μL of ionized water was added to the sample bottle, shaken to dissolve it completely and mixed evenly to obtain the precursor liquid of the hydrogel.
[0045] 3) Preparation of hydrogel: Add 100 μL of glucose (100 mM) solution to the above precursor solution, shake immediately, and mix quickly to obtain a transparent self-supporting hydrogel. The gel formation time About 3s, the compressive modulus is 65.3kPa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com