Method for realizing N-alpha site arylation of nitrogen-containing heterocyclic ring by photocatalysis
A nitrogen-heterocyclic, photocatalytic technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as inability to achieve high efficiency, green and mild reaction conditions, and good substrate universality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
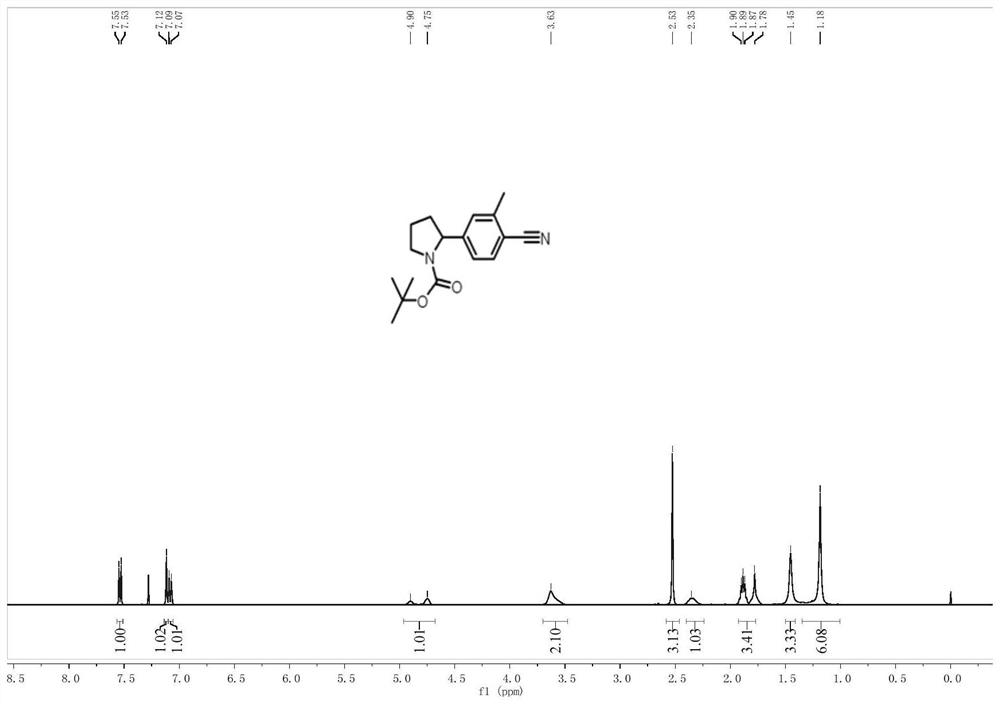
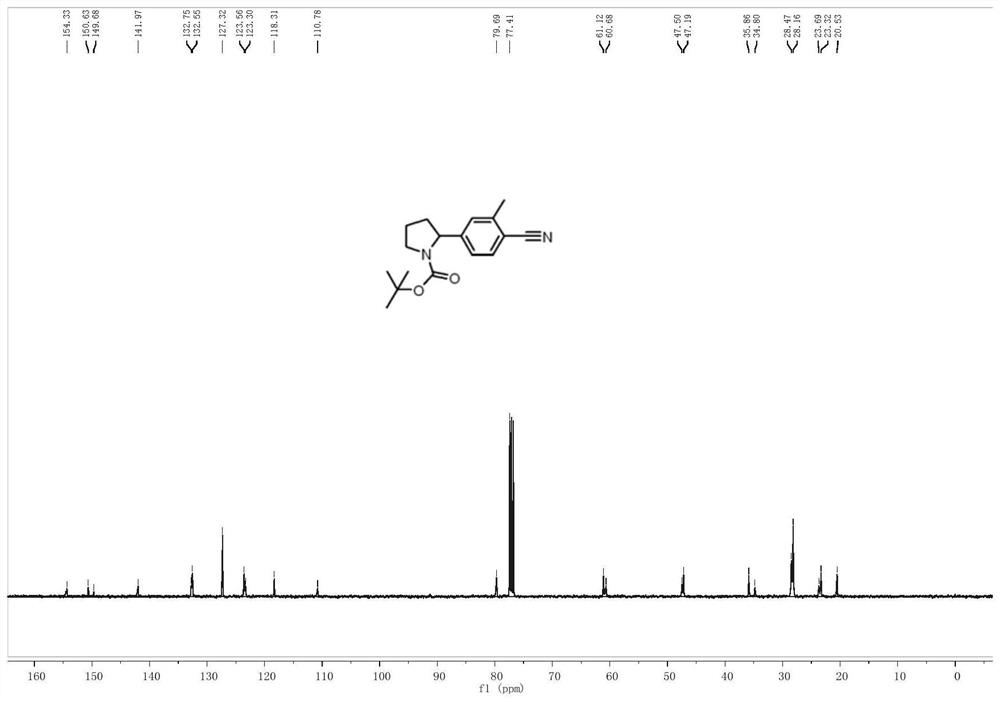
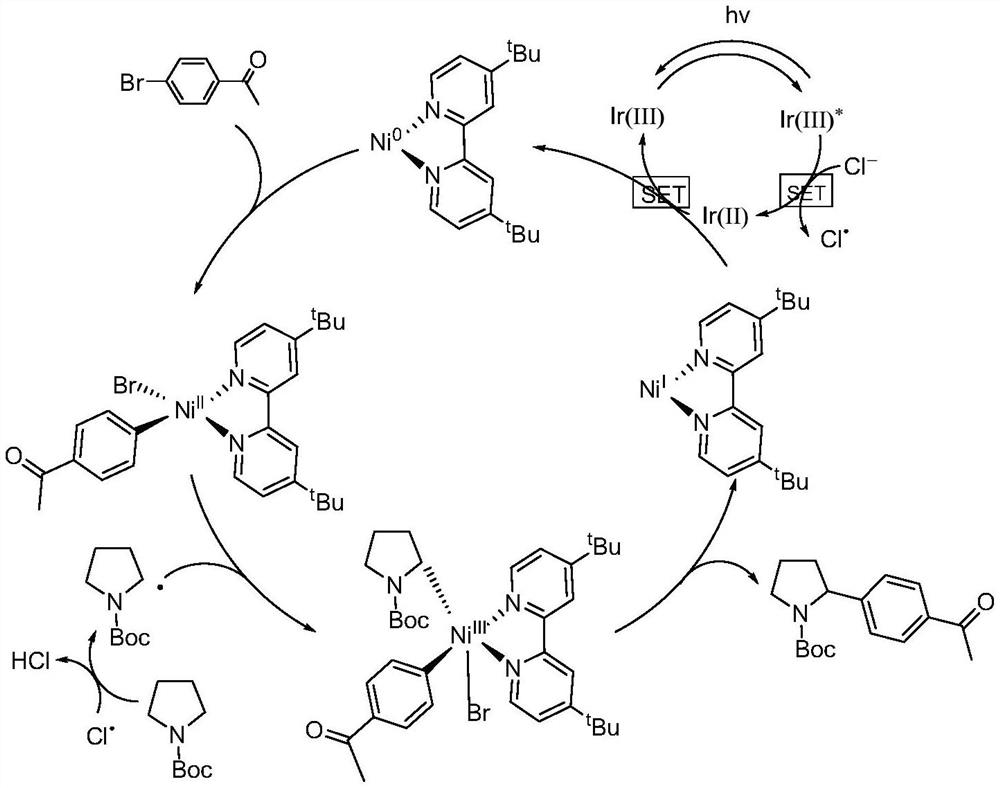
[0032] Embodiment 1: A photocatalytic method for arylation of nitrogen-containing heterocycles at N-α position in this embodiment includes the following steps:
[0033] First, the brominated aromatic compounds, nitrogen-containing heterocyclic compounds, Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 , nickel chloride, 4,4-di-tert-butyl-2,2-bipyridine and alkali are added to the mixed solvent to obtain a mixed solution;
[0034] 2. Under the condition of inert atmosphere and room temperature, use the light source to irradiate the mixed solution obtained in step 1 for 12-20h;
[0035] 3. The mixed solution treated in the second step is subjected to rotary evaporation to remove the solvent, and then separated and purified by thin-layer chromatography. The obtained product is a nitrogen-containing heterocyclic N-α position aryl compound, and the preparation is completed.
specific Embodiment approach 2
[0036] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the brominated aromatic hydrocarbon compounds in step 1 are 4-bromoacetophenone, p-trifluoromethyl bromobenzene, p-methoxybromobenzene, p-bromobenzene Benzaldehyde, p-bromophenylacetonitrile, 2-trifluoromethyl-4-bromopyridine or 2-fluoro-4-bromopyridine. Others are the same as the first embodiment.
specific Embodiment approach 3
[0037] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the nitrogen-containing heterocyclic compounds in step 1 are N-Boc-pyrrolidine, N-Piv-pyrrolidine, N-Ac-pyrrolidine , N-Cbz-pyrrolidine, N-benzoylpyrrolidine, N-Boc-piperidine, N,N-dimethylpropionamide, N-Boc-cycloheptylamine or N-Boc-3-fluoro-pyrrole alkyl. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com