Coxsackie virus A6 type strain and immunogenic composition and application thereof
A coxsackie virus, immunogenic technology, applied in antiviral agents, viruses/phages, biochemical equipment and methods, etc., can solve the problems that CV-A6 strains have not been found, CV-A6 strains cannot grow, etc. , to achieve the effect of strong cross neutralization ability, good immunogenicity and good passive immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1 Isolation and culture of CV-A6 virus strain
[0091] 1. Processing of clinical samples
[0092] The clinical samples were processed according to the guidelines for the prevention and control of hand, foot and mouth disease (2009 edition).
[0093] 2. Virus isolation
[0094] The processed samples were inoculated into 80~90% density of healthy non-polluting African green monkey kidney passage cells (Vero) according to a certain proportion, 35 ℃, 5% CO. 2 Adsorbed in the incubator for more than half an hour, replenished to the culture volume, 35°C, 5% CO 2 The cells were cultured in an incubator, and a cell control without sample was set at the same time. Use an inverted microscope to observe cells every day, such as enterovirus cytopathic effect (CPE): cells become rounded, refraction enhanced and detached from the tube wall, etc., record the changes, and continuously observe the changes in the inoculated wells and control wells within 7 days. CPE (1+~4+). ...
Embodiment 2
[0097] Example 2 Identification and detection of virus strains
[0098] Molecular identification, genome sequencing and titer detection were performed on the fourth-generation virus liquid of Coxsackie virus A6 strain V991 harvested in Example 1.
[0099] 1. Molecular identification and genome sequencing
[0100] The virus was identified by RT-PCR, and the general primers and VP1-specific primers used for enterovirus nucleic acid detection are shown in Table 1.
[0101] Table 1 Universal primers and VP1-specific primers for enterovirus nucleic acid detection
[0102]
[0103] (1) Virus nucleic acid extraction
[0104] Take the fourth-generation virus solution, add reagents and virus samples according to the instructions, then place it in a nucleic acid extractor, extract nucleic acids according to the preset procedure, and store the extracted nucleic acids in a -70°C refrigerator.
[0105] (2) PCR detection of HEV-5UTR universal primers
[0106] Nested PCR was performed...
Embodiment 3
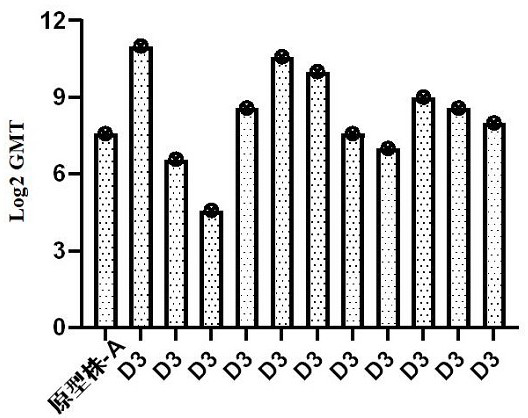
[0140] Example 3 Detection of cross-neutralization ability of CV-A6 strains
[0141] The immune serum of Coxsackie virus A6 strain V991 (hereinafter referred to as CV-A6 serum) was tested for cross-neutralization ability by microcytopathic method. The specific method is as follows: after inactivating the serum in a water bath at 56 °C for 30 minutes, dilute it from 1:8, add it to a 96-well plate, 2 wells per sample, 100 μl / well, after 2-fold dilution, add 32~320 CCID 50 / 0.05ml of virus solution at 36±1℃, 5% CO 2 Incubator, neutralize for 1 to 2 hours. Add RD cell suspension (1~2 × 10 5 pcs / ml), 100 μl / well. Put in 5% CO 2 After culturing for 7 days, the results were judged. Neutralization titers < 8 were judged negative, and ≥ 8 were positive.
[0142] The immune sera of Coxsackie virus A6 strain V991 were cross-neutralized with 12 different CV-A6 strains, of which 1 was the prototype strain (type A) and 11 were circulating genotype (D3) (cross-neutralization). And the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com