Colorimetric reagent for detecting urotropin
A technology of urotropine and reagents, which is applied in the field of colorimetric reagents for detecting urotropine, can solve problems such as unsuitable analysis and detection, and achieve the effects of stable and clear color development, strong anti-interference, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] At room temperature, 15 mg of Fast Blue B fluoroborate was weighed and dissolved in 30 mL of ethanol, and ultrasonicated for 10 min until uniform, to obtain a colorimetric reagent for detecting non-standard explosive raw material urotropine.
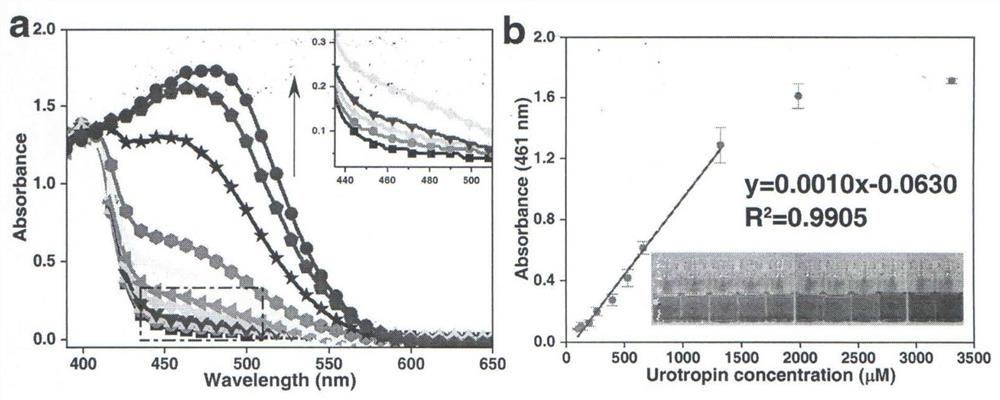
[0021] Drop 20 μL of the aqueous solution of urotropine to be tested into 3 mL of the detection reagent to obtain a mixed solution with a concentration of 265 μM of urotropine, and the change of the solution from pale yellow to bright yellow can be observed within 2s ( figure 1 ), indicating that there is the presence of unstandardized explosive raw material urotropine in the test material, the reaction speed is fast, the color recognition is obvious, and the color development is stable.
Embodiment 2
[0023] At room temperature, 7 mg of Fast Blue B zinc chloride salt was weighed and dissolved in 30 mL of ethylene glycol methyl ether, and ultrasonicated for 10 min until uniform, to obtain a colorimetric reagent for detecting non-standard explosive raw material urotropine.
[0024] Drop 20 μL of the aqueous solution of urotropine to be tested into 3 mL of the detection reagent to obtain a mixed solution with a concentration of 662 μM of urotropine. The change of the solution from light yellow to orange can be observed within 2s ( figure 1 ), indicating that there is the presence of unstandardized explosive raw material urotropine in the test material, the reaction speed is fast, the color recognition is obvious, and the color development is stable.
Embodiment 3
[0026] At room temperature, 43 mg of Fast Blue B fluoroborate was weighed and dissolved in 30 mL of methanol, and ultrasonicated for 10 min until uniform, to obtain a colorimetric reagent for detecting non-standard explosive raw material urotropine.
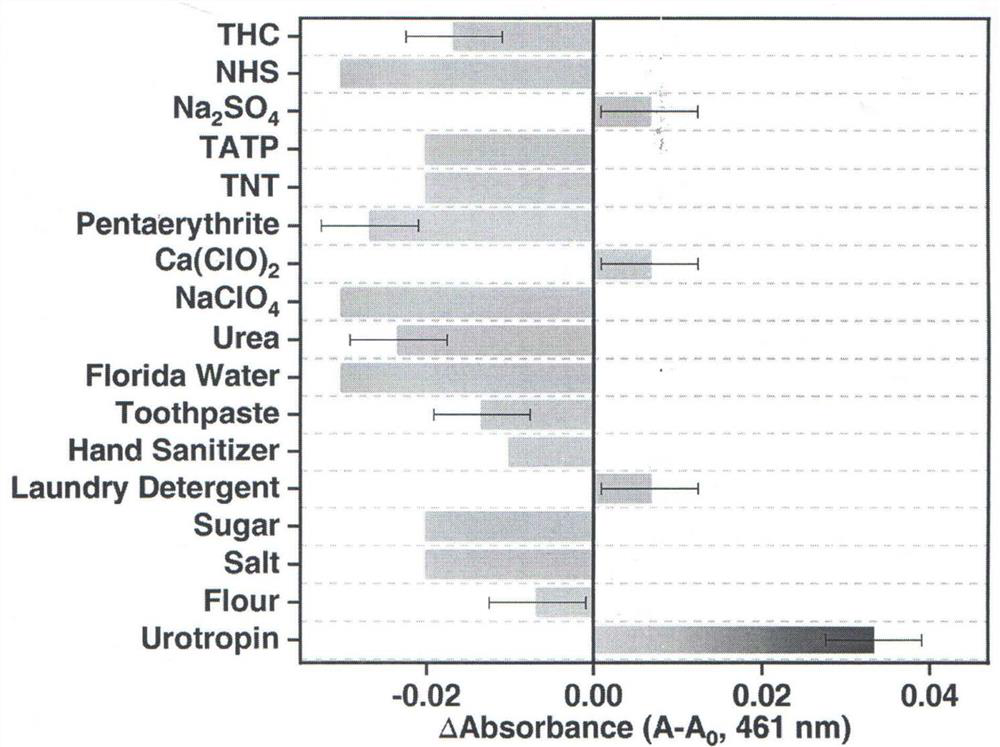
[0027] Drop 20μL solution of interfering substances that may coexist with urotropine (including daily necessities, explosive materials or explosives and structural similarities) solution into 3mL of detection reagent, the difference between the absorption intensity at 461nm and the difference before detection The values indicate that none of the interfering substances interfered with the detection of urotropine. Among them, common daily necessities include flour, salt, sugar, laundry detergent, hand sanitizer, toothpaste and toilet water, and explosive materials or explosives include sodium perchlorate, calcium hypochlorite, pentaerythritol, 2,4,6-trinitro Toluene (TNT) and triacetone triperoxide (TATP), structural analogs incl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com