Reduction-responsive viologen molecule, polypeptide-viologen derivative, preparation method and application
A derivative, responsive technology, applied in the field of biomedicine, can solve problems such as deficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] A method for synthesizing a reduction-responsive viologen molecule, comprising the following steps:
[0074] (1)C n V + Synthesis:
[0075] ① Dissolve 200 mg of 4,4'-bipyridine in 10 ml of dichloromethane, pass nitrogen to remove oxygen, add 80 μL of methyl iodide dropwise under nitrogen atmosphere, and react in the dark for 24 hours at room temperature; after the reaction is completed, filter and collect the filtered cake, washed three times with dichloromethane, and dried in vacuo to give a pale yellow solid C 1 V + ;
[0076] ② Dissolve 200 mg of 4,4'-bipyridine in 10 ml of dichloromethane solution, pass nitrogen to remove oxygen, add 120 μL of n-bromopropane dropwise under nitrogen atmosphere, and react at room temperature in the dark for 24 hours; filter and collect the filter cake , washed three times with dichloromethane, and dried in vacuo to give a yellow solid C 3 V + ;
[0077] ③ Dissolve 200 mg of 4,4'-bipyridine in 15 ml of anhydrous acetonitrile so...
Embodiment 2
[0081] A preparation method of a polypeptide-viologen derivative, comprising the following steps:
[0082] (1) Dissolve 40mg polypeptide molecule Pep in 10mL deionized water, and add 20mg C n V 2+ Mal, dissolved, mixed, and reacted at room temperature for 24 hours; the specific sequence of the polypeptide molecule Pep is NH 2 -C-VAQLEVKVAQLESK-VSKLESK-VSSLESK-COOH;
[0083] (2) the mixed solution obtained in (1) is placed in a dialysis bag, the dialysis bag has a molecular weight cut-off of 3500, and the dialysis bag is put into ultrapure water for dialysis, fresh dialysate is replaced every 2 hours, and dialysis is performed for 24 hours;
[0084] (3) After the dialysis, freeze-dry the solution in the dialysis bag to finally obtain a white loose powder, which is the polypeptide-viologen derivative Pep-C n V 2+ (n=1,3,5).
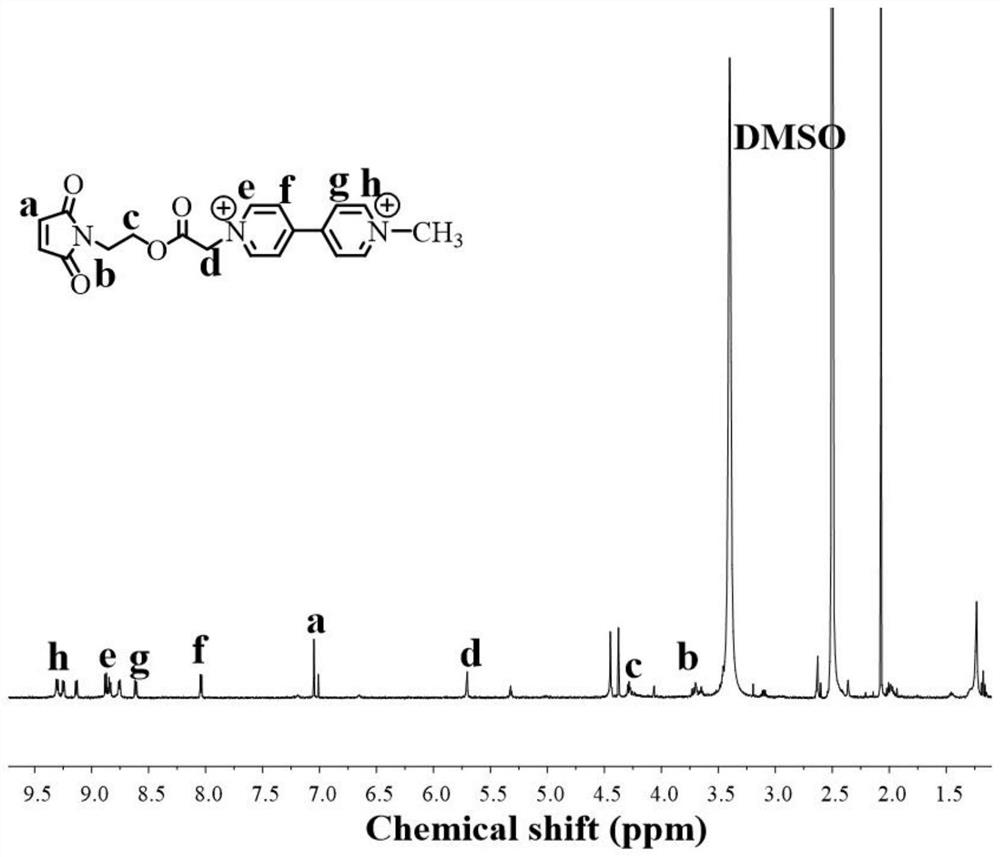
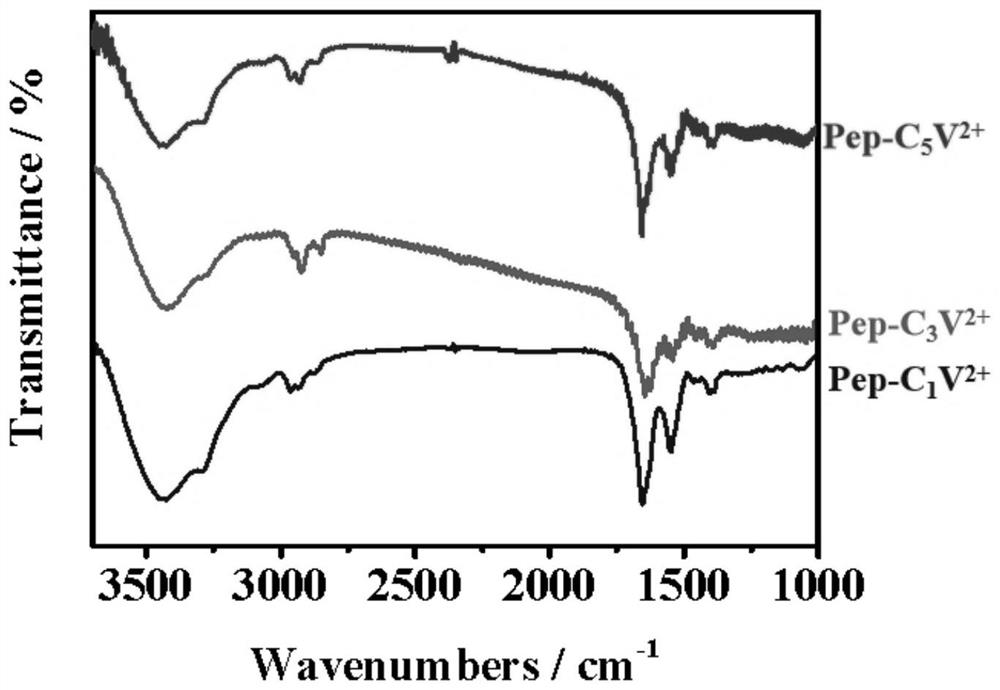
[0085] Determination of C by 1H NMR 1 V 2+ The molecular structure of Mal, as figure 1 shown; the use of infrared spectroscopy to determine the pol...
Embodiment 3
[0086] Example 3 to the polypeptide-viologen derivative Pep-C prepared in Example 2 n V 2+ Conduct reduction responsiveness studies
[0087] 1. Ultraviolet absorption spectroscopy to study reduction responsiveness
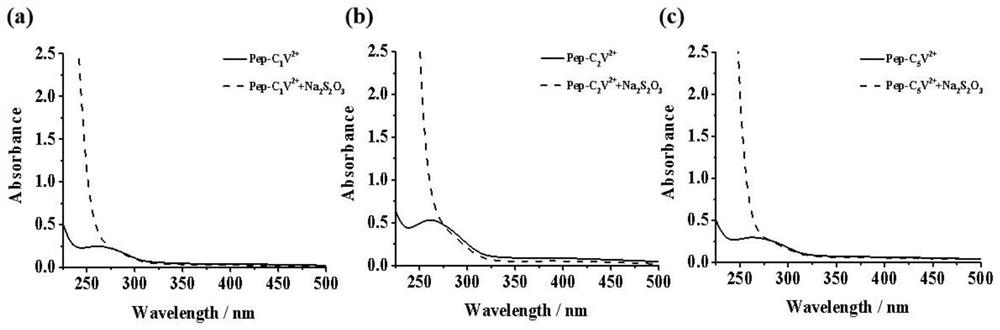
[0088] Prepare 0.1mol of Pep-Cn V 2+ The aqueous solution was measured by adding Na at room temperature 2 S 2 O 3 Before and after UV absorption spectrum (200nm-500nm), among which, Pep-C n V 2+ with Na 2 S 2 O 3 The mass ratio is 1:9. like image 3 shown, in Na 2 S 2 O 3 Before adding, a characteristic absorption peak appeared at 260 nm; by contrast, adding Na 2 S 2 O 3 After that, the characteristic peak at 260nm disappeared, which proved that the polypeptide-bauhinia derivative had good reduction responsiveness.
[0089] 2. Fluorescence spectroscopy to study reduction responsiveness
[0090] Prepare 0.1mol of Pep-C n V 2+ The aqueous solution was measured by adding Na at room temperature 2 S 2 O 3 Fluorescence spectrum before and after (4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com