Method for preparing glycollic acid and methyl glycolate by hydrolyzing methyl methoxyacetate and methoxyacetic acid
A technology of methyl methoxyacetate and methoxyacetic acid, which is applied in the field of chemical product preparation, and can solve the problems of waste of raw materials and no application of preparation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0075] According to the problems existing in the existing production technologies of glycolic acid and methyl glycolate, the present invention develops a method for preparing glycolic acid and methyl glycolate by hydrolysis of methoxyacetate and methoxyacetic acid. Moreover, the method of the invention is especially suitable for methylal produced by coal chemical industry, and methyl methoxyacetate is generated through carbonylation reaction, and then glycolic acid and methyl glycolate are prepared by hydrolysis.
[0076] Specifically, the present invention provides a method for preparing glycolic acid and methyl glycolate by hydrolysis of methoxyacetate and methoxyacetic acid. The raw materials methoxyacetate, methoxyacetic acid and water are passed through The reaction zone carrying the catalyst is reacted to produce glycolic acid and methyl glycolate under certain reaction conditions;
[0077] The catalyst is any one or a mixture of a solid acid catalyst, a liquid acid cata...
Embodiment 1
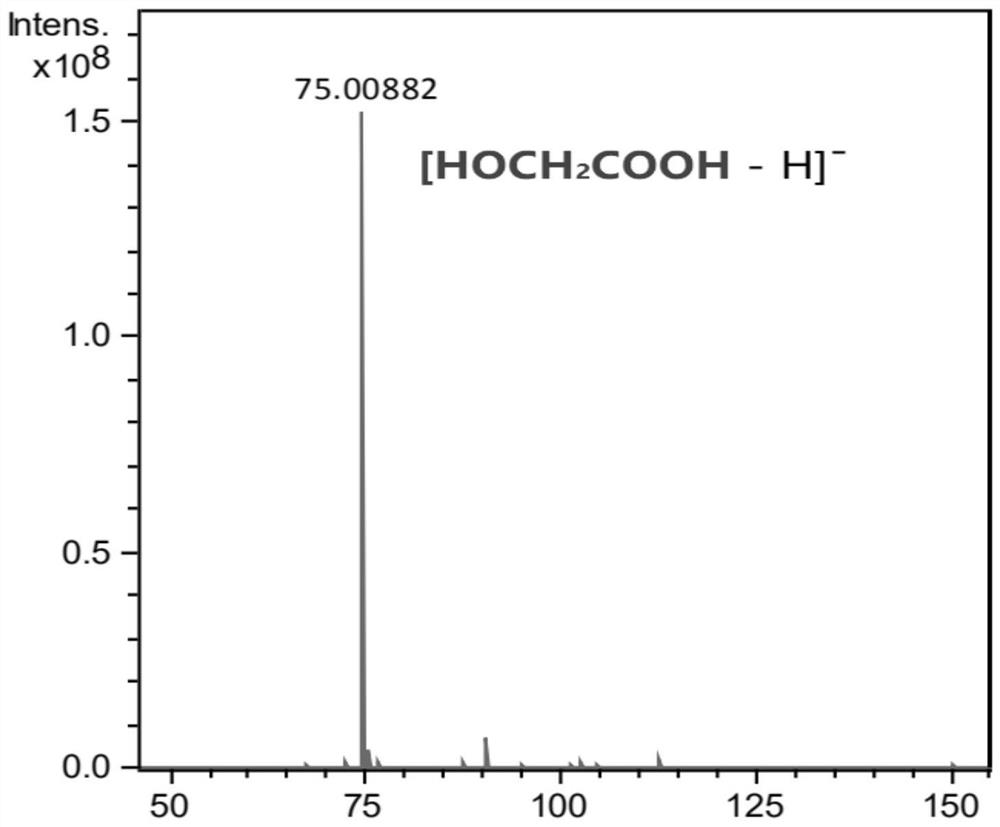
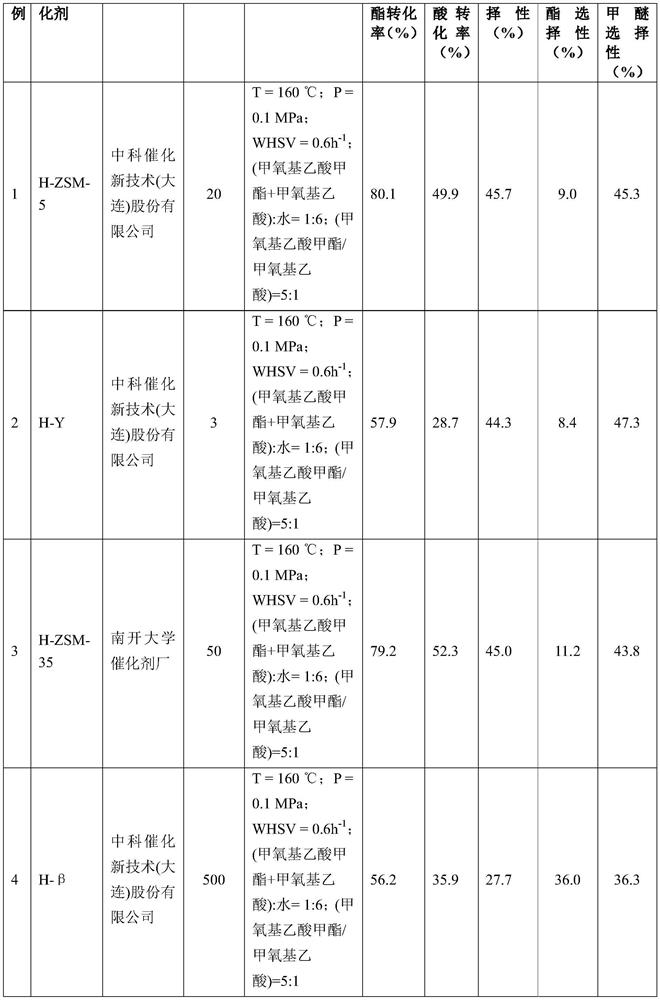
[0118] Select the acid H-ZSM-5 molecular sieve with Si / Al=20 purchased from the Catalyst Factory of Zhongke Catalysis New Technology (Dalian) Co., Ltd., crush and sieve it into 0.4-0.8mm particles, take 2g Into a stainless steel reaction tube with an inner diameter of 8mm, activate it with 50mL / min nitrogen at 500°C for 4h, and react under the following conditions: reaction temperature (T)=160°C, reaction pressure (P)=0.1MPa, raw material molar ratio (methoxyl Methyl acetate + methoxyacetic acid) / water = 1:6; molar ratio (methyl methoxyacetate / methoxyacetic acid) = 5:1; the mass of raw material methyl methoxyacetate and methoxyacetic acid Airspeed (WHSV)=0.6h -1 . After 24 hours of reaction, the product was analyzed by gas chromatography and liquid chromatography. The reaction results based on carbon number are shown in Table 1. Among them, the negative ion mass spectrometry results of glycolic acid in the product analyzed by liquid chromatography-mass spectrometry are as fo...
Embodiment 2-8
[0120] The catalysts, reaction conditions and reaction results are shown in Table 1. Other operations are the same as in Example 1.
[0121] Catalytic reaction results in Table 1 Examples 1-8
[0122]
[0123]
[0124]
[0125] It can be seen from Table 1 that the acidic molecular sieves show good catalytic performance in the reaction of methyl methoxyacetate and methoxyacetic acid to hydrolyze glycolic acid and methyl glycolate, and the target product selectivity is high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com