Method for culturing human primary cells
A culturing method and primary cell technology are applied in the field of culturing human-derived primary cells to achieve the effects of reducing the amount of bacteria, improving the success rate and reversing bacterial contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
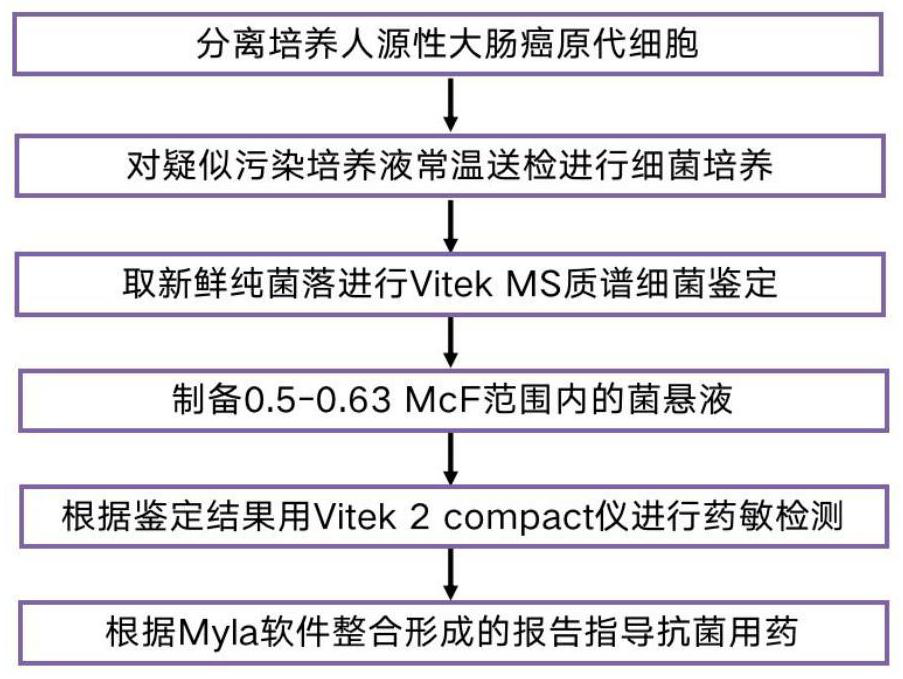
[0040] Example 1 Isolation and culture of primary cells
[0041] A fresh human colorectal tumor specimen of about 0.5 × 0.5 cm in size was taken from surgical resection, and the adipose tissue was trimmed, immediately placed in PBS solution with penicillin and streptomycin, aseptically sealed, and sent to the laboratory at low temperature. Irradiate the ultra-clean bench with UV light for 30 minutes, turn on the fan for self-cleaning for 3 minutes, rinse the tumor tissue with sterile PBS three times in the sterile ultra-clean bench, discard the supernatant, add sterile penicillin, streptomycin and a small amount of sterile cell culture The solution was placed in a petri dish, and the specimen was cut into tissues of approximately 0.03-0.08 mm in size with sterile ophthalmic scissors. Using human tumor tissue separation reagent (purchased from Miltenyi Bioted), digest and disintegrate and make it loose by pipetting, suck the tissue fragments into a 6 cm petri dish, add 4 mL of ...
Embodiment 2
[0042] Example 2 Bacterial identification by Vitek MS mass spectrometry
[0043] Dip the culture solution with a disposable inoculating loop and inoculate it on the blood agar plate, the chocolate agar plate and the MacConkey plate, respectively, and place it on the blood agar plate, the chocolate agar plate and the MacConkey plate. %CO 2 After incubation in the incubator for 18-24 hours, the bacterial colonies were observed for growth and purified.
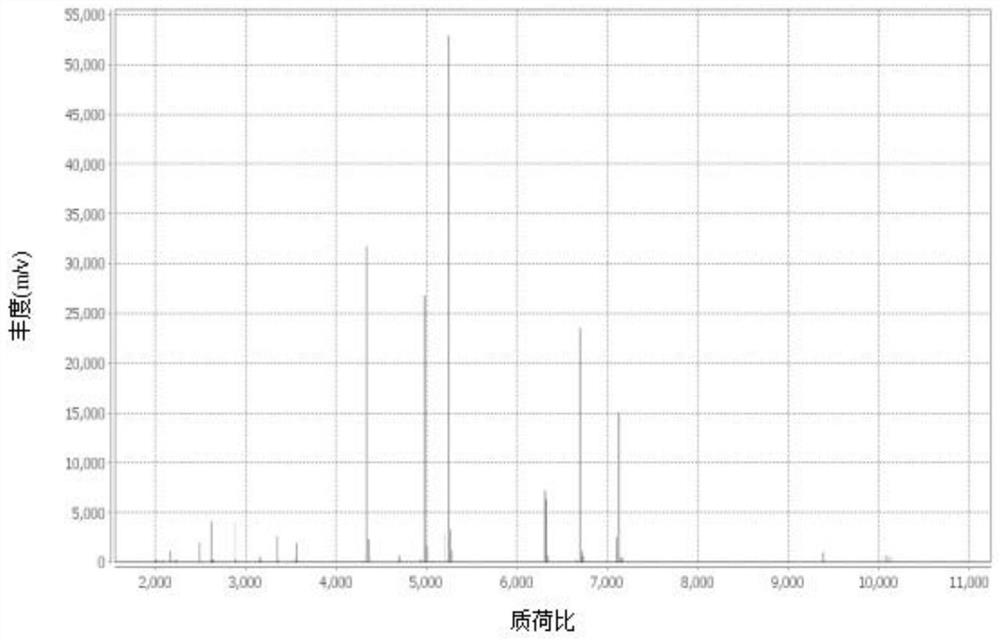
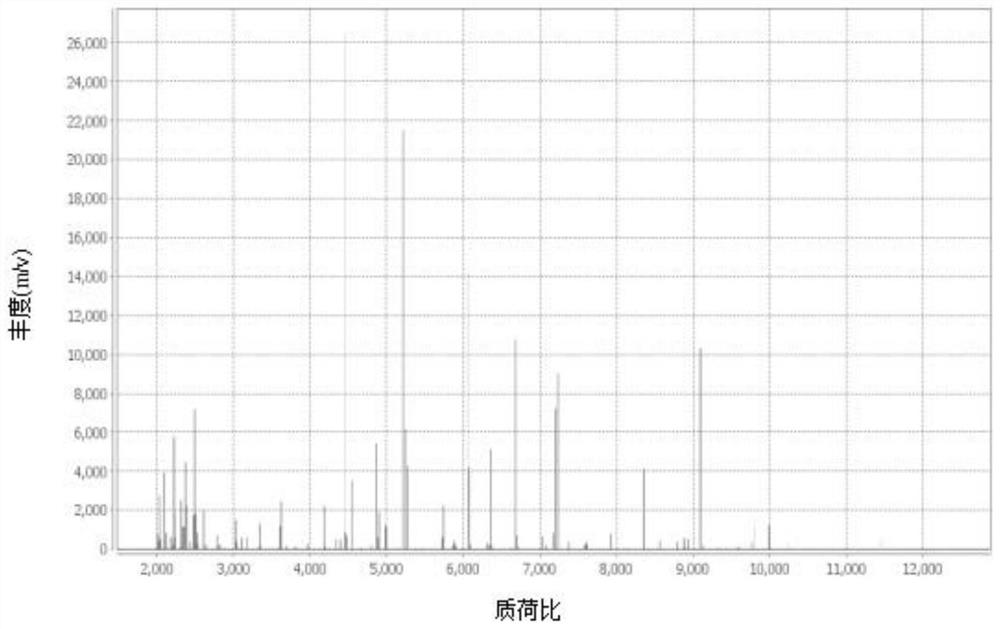
[0044] Pick a single purified colony, apply 0.95 μl of Vitek MS-CHCA matrix to the target plate, and then dry at room temperature; if the bacteria are suspected of having cell walls, add 0.45 μl of 25% formic acid first, and then add 0.95 μl of Vitek MS-CHCA matrix after drying at room temperature. Input the sample information into the mass spectrometer workstation, put the target plate into the Vitek MS instrument for MS detection, obtain the MALDI-TOF peak map, and match the bacterial species to Achromobacter xyloseoxidizing a...
Embodiment 3
[0045] Example 3 Drug susceptibility detection by Vitek 2compact instrument
[0046] Remove the 0.45% NaCl bottle from the refrigerator and return to room temperature. Take a disposable transparent plastic test tube, add 3 ml of sterile 0.45% NaCl to each tube, adjust the pH to 7.0, pick colonies and dissolve them into the saline in the test tube. Test with a turbidimeter, and adjust the turbidity of the bacteria test tube within the range of 0.5-0.63McF.
[0047] Achromobacter xyloseoxidizing bacteria and Pseudomonas aeruginosa were Gram-negative bacteria, and Gram-negative bacteria drug susceptibility card N335 was selected for drug susceptibility testing of the two strains. Before use, take the drug susceptibility card out of the refrigerator, return to room temperature, place the bacterial suspension test tube and the drug susceptibility card in sequence on the card carrier, and insert the catheter of the drug susceptibility card into the bacterial suspension. Enter the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com