Metal phosphatase as well as carrier, storage solution and preparation method thereof
A metallophosphatase and amino acid technology, applied in the field of molecular biology, can solve the problem of stable expression of metallophosphatase and achieve broad market application prospects, short production cycle and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation method, expression gene, recombinant expression vector and vector construction method of recombinant metallophosphatase AMUC_1901
[0045] The species source of the metallophosphatase in this example is: Akkermansia muciniphila strain ATCC BAA-835 B2UNJ9
[0046] The Uniprot number is: B2UNJ9_AKKM8. The amino acid sequence of the metallophosphatase in this example is shown in SEQ ID No. 1, and the nucleotide sequence encoding the metallophosphatase gene is shown in SEQ ID No. 2.
[0047] In this example, the part of the amino-terminal signal peptide sequence was removed by artificially synthesizing the full-length gene sequence, and then it was linked into the vector pMAL-c5X, and the enzyme cleavage sites were NdeI and PstI. The Amuc_1901 gene was cloned from the genome of AKK bacteria using specific primers for the Amuc_1901 gene, and PCR was performed using a high-fidelity enzyme from NEB.
[0048] The plasmid vector pMAL-c5X-Amuc_1901 was c...
Embodiment 2
[0050] Example 2 Expression of metallophosphatase
[0051] The positive clones expressing metallophosphatase obtained by transformation were picked, and the recombinant expression strain was inoculated into 5 ml of liquid ampicillin LB medium, and cultured with shaking at 37° C. and 220 rpm for 5-6 hours.
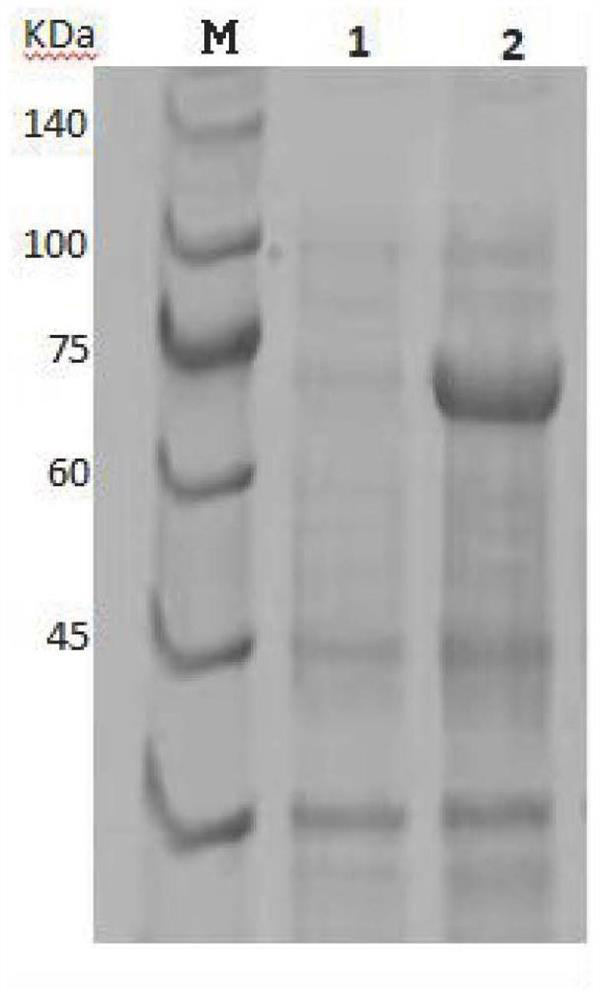
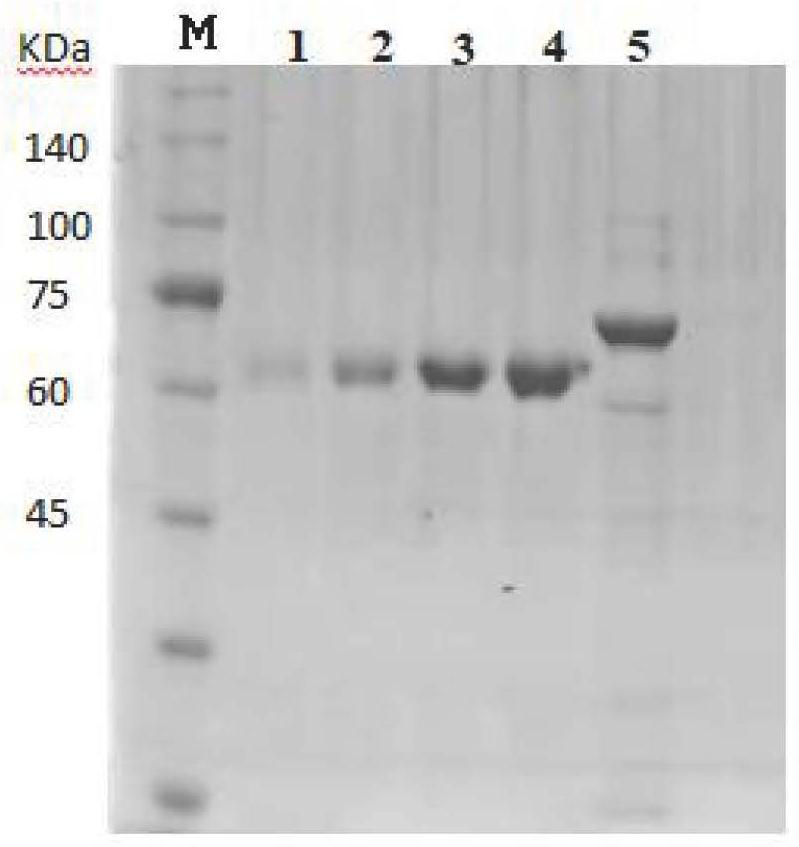
[0052] The strain culture liquid was transferred to 200 ml of LB liquid medium, and after 6 hours of shaking culture at 37° C. and 220 rpm, cultured to OD=0.6-0.8. The inducer IPTG was added to a final concentration of 0.5 mM and incubated overnight at 16°C with shaking at 180 rpm. The cells were collected and centrifuged at 8000 rpm for 15 min. Resuspend the cell pellet with 50mM Tris-HCl pH8.0 and 200mM NaCl, lyse for 10min in a homogenizer at 850MPa-900MPa, centrifuge the lysate at 20000g for 20min at high speed, and take the supernatant. After filtration with a 0.45um membrane, the lysed supernatant was loaded into a Ni affinity chromatography column, incubated at 4°C...
Embodiment 3
[0053] Example 3 Determination of Metallophosphatase Activity and Biochemical Index Verification
[0054] The metallophosphatase prepared in Example 2 is stored in the storage solution of metallophosphatase, and the formula of this storage solution is: 1×PBS buffer (Na2HPO41.42g, KCl0.2g, NaCl 8g, KH2PO40.27g weigh each group Divide it into a 1L beaker, add 800mL deionized water to the beaker, stir and dissolve with a glass rod, dilute to 1L, add NaOH or dilute hydrochloric acid to adjust the pH to 7.4) and add 50% glycerol to obtain a total of 10ml of finished metallophosphatase.
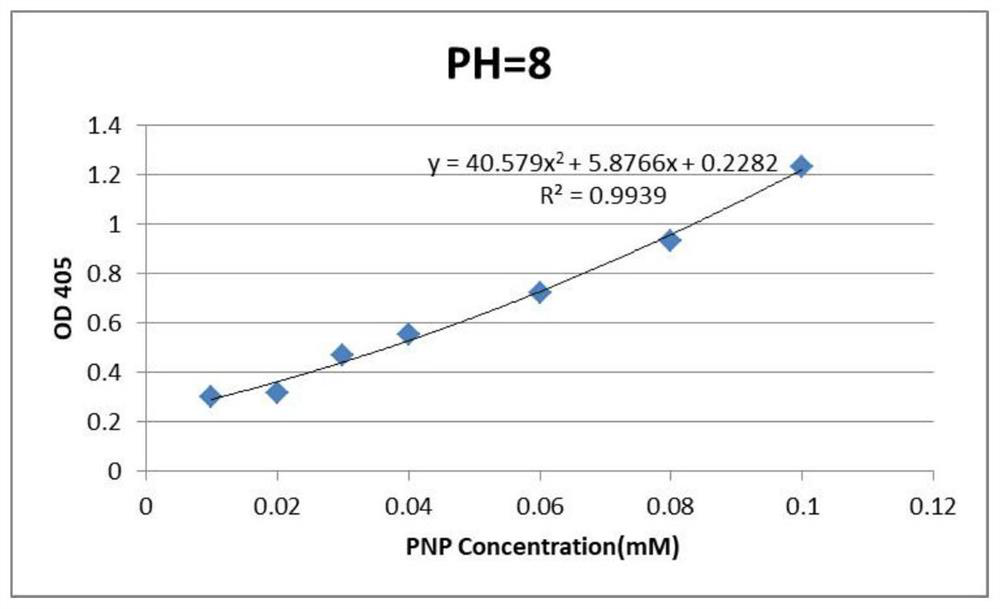
[0055] Using p-nitrophenyl phosphate monoesters as the substrate, metallophosphatase is used to break the phosphodiester bond between the PNP (p-nitrophenyl) head group and the phosphate ester to release PNP, wherein PNP has a maximum absorption peak at OD410, After the PNP standard curve was drawn, the absorbance value of PNP was detected, which could reflect the hydrolysis rate of p-nitrophenylph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com