Polymer electrolyte based on boric acid ester exchange reaction as well as preparation method and application of polymer electrolyte
An exchange reaction and polymer technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., to achieve the effects of promoting lithium salt dissociation, enhancing exercise capacity, and improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
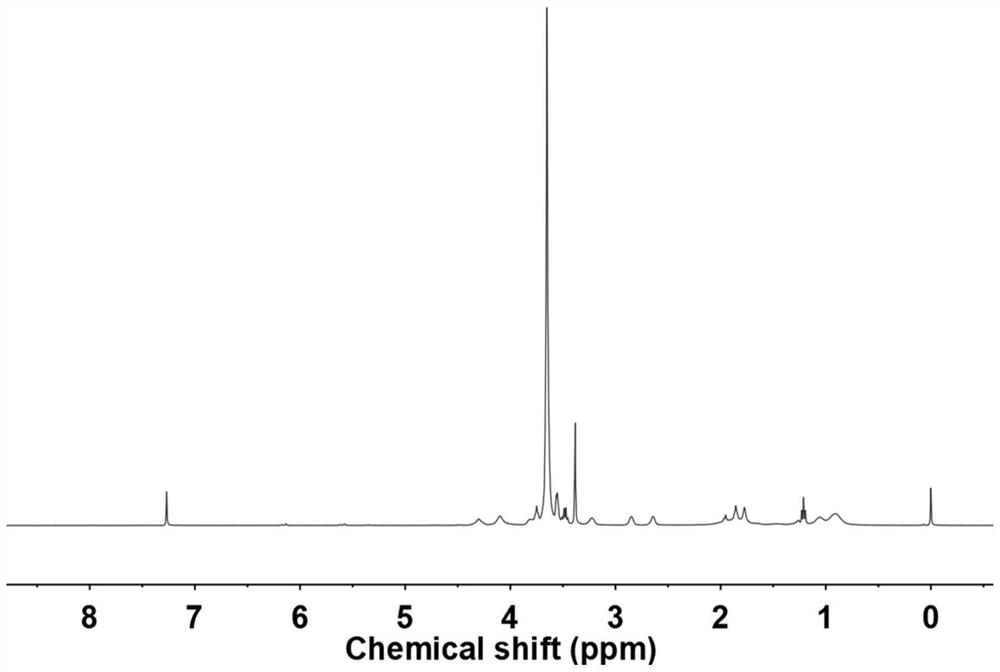
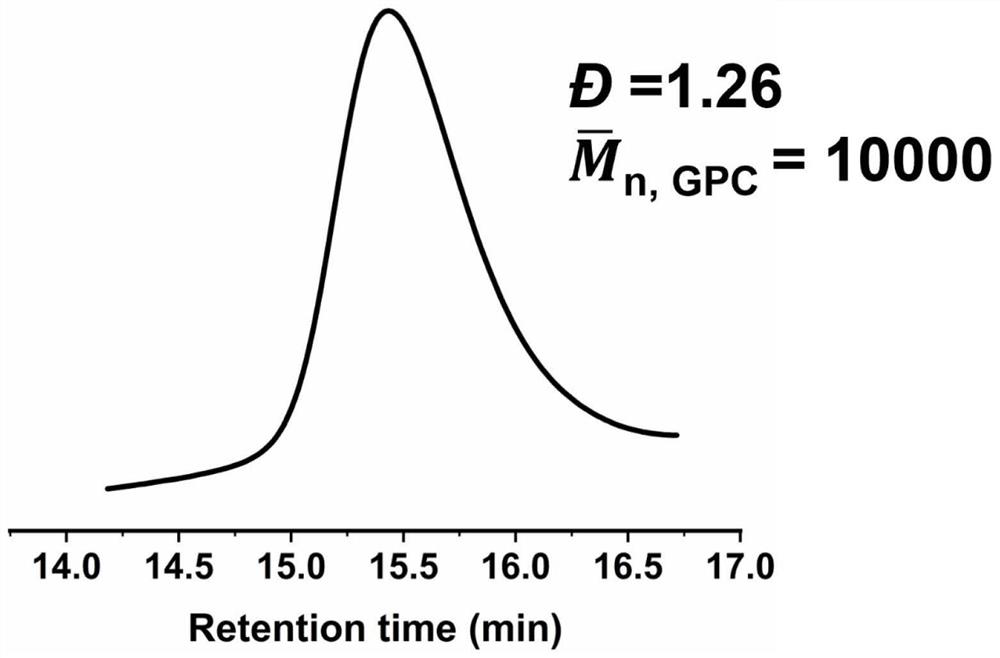
[0043] The preparation method of the conductivity-enhanced polymer electrolyte based on borate exchange provided in this embodiment is as follows;
[0044] S1: 1.425g polyethylene glycol methyl ether methacrylate with an average molecular weight of 475 ( n=9, the manufacturer is Aladdin Reagent Company) and 0.48g 2-methyl-2-acrylic acid-2,3-dihydroxypropyl ester, 16.74mg dithiobenzoic acid 4-cyanovaleric acid, 3.28mg azo Diisobutyronitrile, dissolved in 5 mL of tetrahydrofuran, removed the oxygen in the reaction flask, heated to 60 °C for 24 hours, precipitated the reacted solution in ether, and used an oil pump to remove the ether to obtain the main chain of the copolymer;
[0045] S2: 0.75g of 2-formylbenzeneboronic acid ( Belonging to formyl phenylboronic acid PBA) with 10.35g M 2070 (ie, polyetheramine M 2070 ) in 20 mL of ethanol solvent at 30 °C for 24 h, and the solvent was removed by rotary evaporation to obtain PBA M 2070 ;
[0046] S3: Take 0.16 g of the copol...
Embodiment 2
[0052] This embodiment provides a polymer electrolyte with enhanced conductivity based on a boronate exchange reaction and a preparation method thereof is as follows;
[0053] S1: 1.425g polyethylene glycol methyl ether methacrylate with an average molecular weight of 475 ( n=9, the manufacturer is Aladdin Reagent Company) and 0.48g 2-methyl-2-acrylic acid-2,3-dihydroxypropyl ester, 16.74mg dithiobenzoic acid 4-cyanovaleric acid, 3.28mg azo Diisobutyronitrile, dissolved in 5 mL of tetrahydrofuran, removed the oxygen in the reaction flask, heated to 60 °C for 24 hours, precipitated the reacted solution in ether, and used an oil pump to remove the ether to obtain the main chain of the copolymer;
[0054] S2: 0.75g of 2-formylphenylboronic acid with 10.35g M 2070 The reaction was carried out in 20 mL of ethanol solvent at 30 °C for 24 h, and the solvent was removed by rotary evaporation to obtain PBA M 2070 ;
[0055] S3: Take 0.16 g of the copolymerized backbone obtained i...
Embodiment 3
[0057] This embodiment provides a polymer electrolyte with enhanced conductivity based on a boronate exchange reaction and a preparation method thereof is as follows;
[0058] S1: 1.425g polyethylene glycol methyl ether methacrylate with an average molecular weight of 475 ( n=9, the manufacturer is Aladdin Reagent Company) and 0.48g 2-methyl-2-acrylic acid-2,3-dihydroxypropyl ester, 16.74mg dithiobenzoic acid 4-cyanovaleric acid, 3.28mg azo Diisobutyronitrile, dissolved in 5 mL of dichloromethane, removed the oxygen in the reaction flask, heated to 60 °C for 24 h, precipitated the reacted solution in diethyl ether, and used an oil pump to remove the diethyl ether to obtain the copolymer;
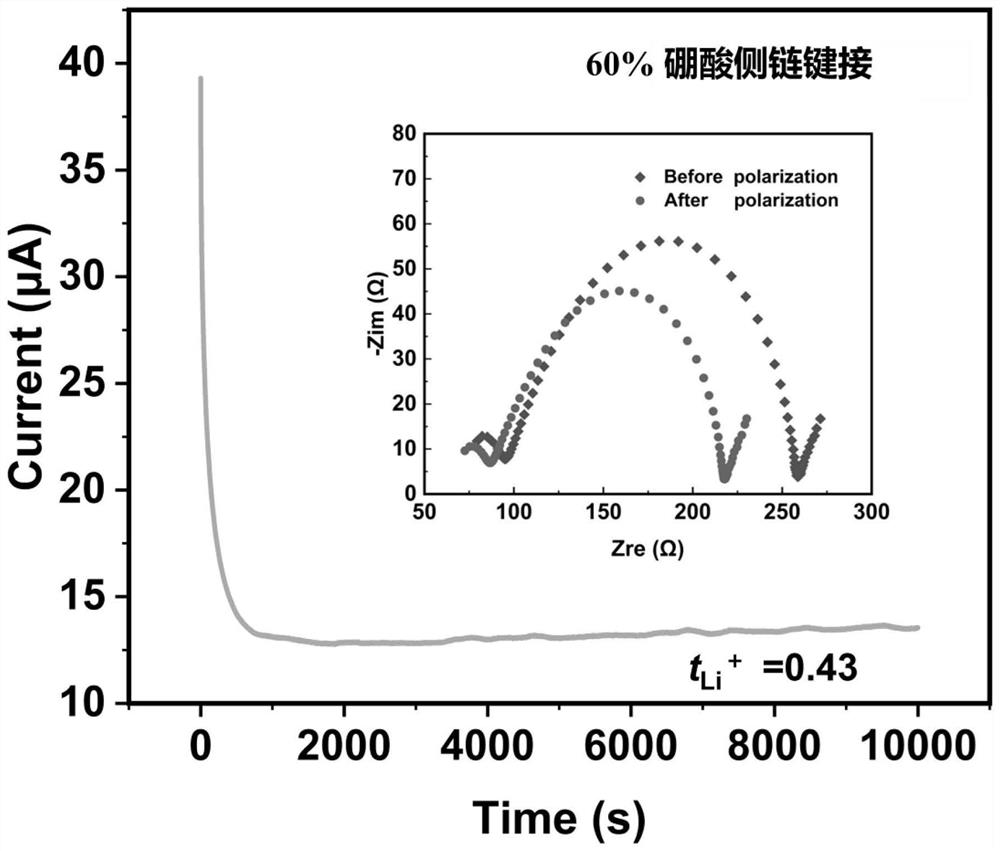
[0059] S2: Take 0.16 g of the copolymer containing polyethylene glycol methyl ether methacrylate and 2-methyl-2-acrylic acid-2,3-dihydroxypropyl obtained in step S1, add 0.016 g of bis-trifluoromethanesulfonyl Lithium imide, according to the lithium salt accounting for 10% of the polymer m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com