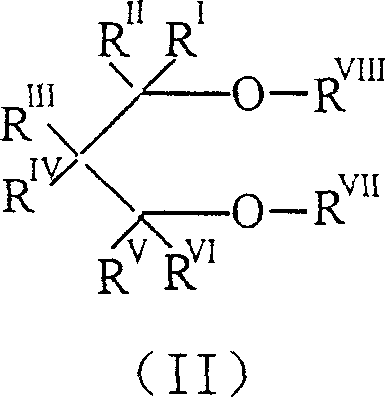
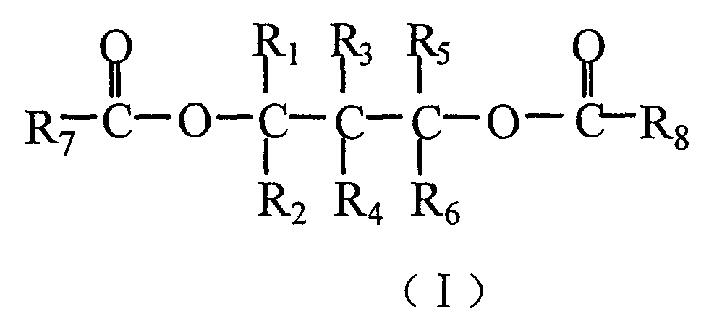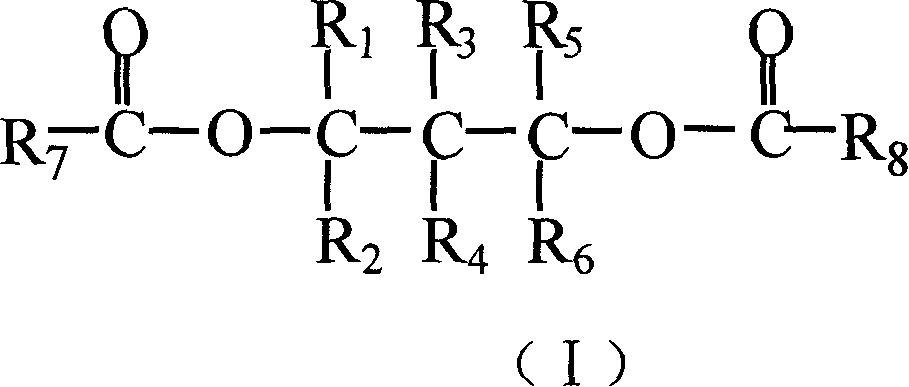Catalyst for olefine polymerizing reaction and its components
A technology for olefin polymerization and catalysts, which is applied in the field of catalyst components and catalysts for olefin polymerization reactions, and can solve the problems of low isotacticity of polymers, narrow molecular weight distribution of polymers, unfavorable polymer development, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of 2,4-pentanediol dibenzoate
[0054] (1) Preparation of 2,4-pentanediol
[0055] A mixture of 10g of 2,4-pentanedione and 30ml of methanol was added dropwise to a mixed solution of 2.5g of sodium borohydride, 0.1g of sodium hydroxide and 25ml of water at 0-10°C. After the addition, the solvent was removed under reduced pressure and extracted continuously with 40ml of ethyl acetate for 15h. The solvent was removed and column chromatography was performed to obtain 9.4 g of colorless liquid 2,4-pentanediol with a yield of 90%. IR spectrum at 3400cm -1 There is a strong absorption peak at 1700cm -1 There are no absorption peaks on the left and right, which proves that the reduction reaction is complete.
[0056] (2) Preparation of 2,4-pentanediol dibenzoate
[0057] Add 30ml tetrahydrofuran and 7.1g (0.09mol) pyridine to 2,4-pentanediol (3.1g, 0.03mol), add 10.5g (0.075mol) benzoyl chloride under stirring, and heat to reflux for 4h. After cooling, add 20ml...
Embodiment 2
[0060] Synthesis of (2S,4S)-(+)-2,4-pentanediol dibenzoate
[0061] (2S,4S)-(+)-2,4-Pentanediol 3.1g, 30ml tetrahydrofuran and 0.09mol pyridine were added to 0.03mol, 0.075mol benzoyl chloride was added under stirring, and heated to reflux for 4 hours. After cooling, add 20ml of saturated brine, extract with ethyl acetate, anhydrous Na 2 SO 4 Dry and remove solvent. Column chromatography gave 8.9 g of (2S,4S)-(+)-2,4-pentanediol dibenzoate as a colorless liquid, with a yield of 95%.
[0062] 1 H NMR (TMS, CDCl 3 , ppm): δ1.2~1.4 (8H, m, methyl H), 2.0~2.1 (2H, m, methylene H), 5.2~5.3 (2H, m, methine H of ester group), δ7.3~8.0 (10H, m, benzene ring H).
Embodiment 3
[0064] Synthesis of (2R,4R)-(+)-2,4-pentanediol dibenzoate
[0065] The method is the same as that of Preparation Example 2, except that (2R,4R)-(+)-2,4-pentanediol is used instead of (2S,4S)-(+)-2,4-pentanediol. 1 H NMR (TMS, CDCl 3 , ppm): δ1.3~1.4 (8H, m, methyl H), 2.0~2.1 (2H, m, methylene H), 5.2~5.3 (2H, m, methine H of ester group), 7.3-8.0 (10H, m, benzene ring H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com