High barrier polyester/phenylenedi (oxyacetic acid) polyester blends
A technology of polyethylene terephthalate and glycolic acid, which is applied in the field of polyester compositions and can solve problems such as poor crystallinity and low gas barrier properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
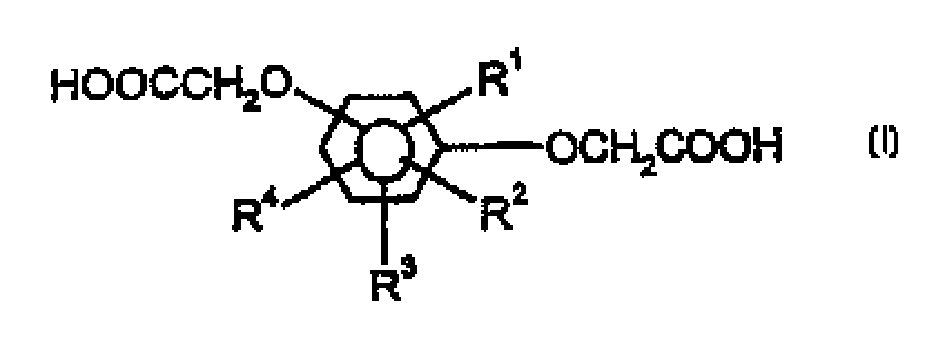
[0047] The preparation of benzenedi(glycolic acid) monomers is disclosed in US 4,935,540, the contents of which are incorporated herein by reference.
[0048] The polyester (I or II) of the present invention preferably has an intrinsic viscosity of 0.4 to 2.0, more preferably 0.50 to 1.2 [measured at 25° C. using a mixed solvent of phenol and tetrachloroethane (weight ratio 60:40)]. If the intrinsic viscosity is less than 0.4, the strength of the resulting polyester is so low that the necessary physical properties cannot be practically obtained when the polyester is discharged from the reactor after polymerization and cut into small pieces. On the other hand, if the intrinsic viscosity is greater than 2.0, the melt viscosity is so high that subsequent processing is difficult.
[0049] The polyester (I or II) of the present invention can be prepared by any generally known polymerization method for the polymerization of polyethylene terephthalate. For example, a polycondensatio...
example
[0087] The following examples are presented in order to provide those of ordinary skill in the art with a complete disclosure and description of how to make the polyester blend of the claimed invention and how to evaluate it, without limiting the scope of the invention as the inventors regard it. Efforts have been made to ensure accuracy with respect to numbers (eg, amounts, temperature, etc.), but some errors and deviations should be accounted for. Unless indicated otherwise, parts are parts by weight, temperature is in °C or is at room temperature, and pressure is at or near atmospheric.
[0088] The invention is illustrated in more detail with reference to the following non-limiting examples.
[0089] Intrinsic viscosity (IhV) measurements were carried out at 25°C in a 60 / 40 (W / W) phenol / tetrachloroethane solvent system.
[0090] The oxygen transmission rate of polyester is measured by MOCON Oxtran 100 instrument, which is the number of cubic centimeters permeated by the f...
example 1
[0093] Poly[1,4-phenylenedi(glycolic acid)ethylene glycol] was prepared as follows. A reaction kettle was charged with 22.42 g of 1,4-benzenedi(glycolic acid), 24.60 g of ethylene glycol, and 100 ppm titanium from titanium isopropoxide. The reaction mixture was heated and stirred at 210 °C under nitrogen atmosphere for 60 min. Then, the temperature was raised to 220° C. for 120 min until all the water was distilled from the reaction mixture. Then, the temperature was raised to 260° C., nitrogen was evacuated from the reaction system, and a vacuum was drawn. Under the column pressure of 0.5mmHg, continue to melt and condense at 260°C for 75min. Heating was discontinued, the reaction mixture was placed under 1 atmosphere of nitrogen, and the polymer was collected. The resulting polymer had an intrinsic viscosity of 0.88 dl / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition point | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com