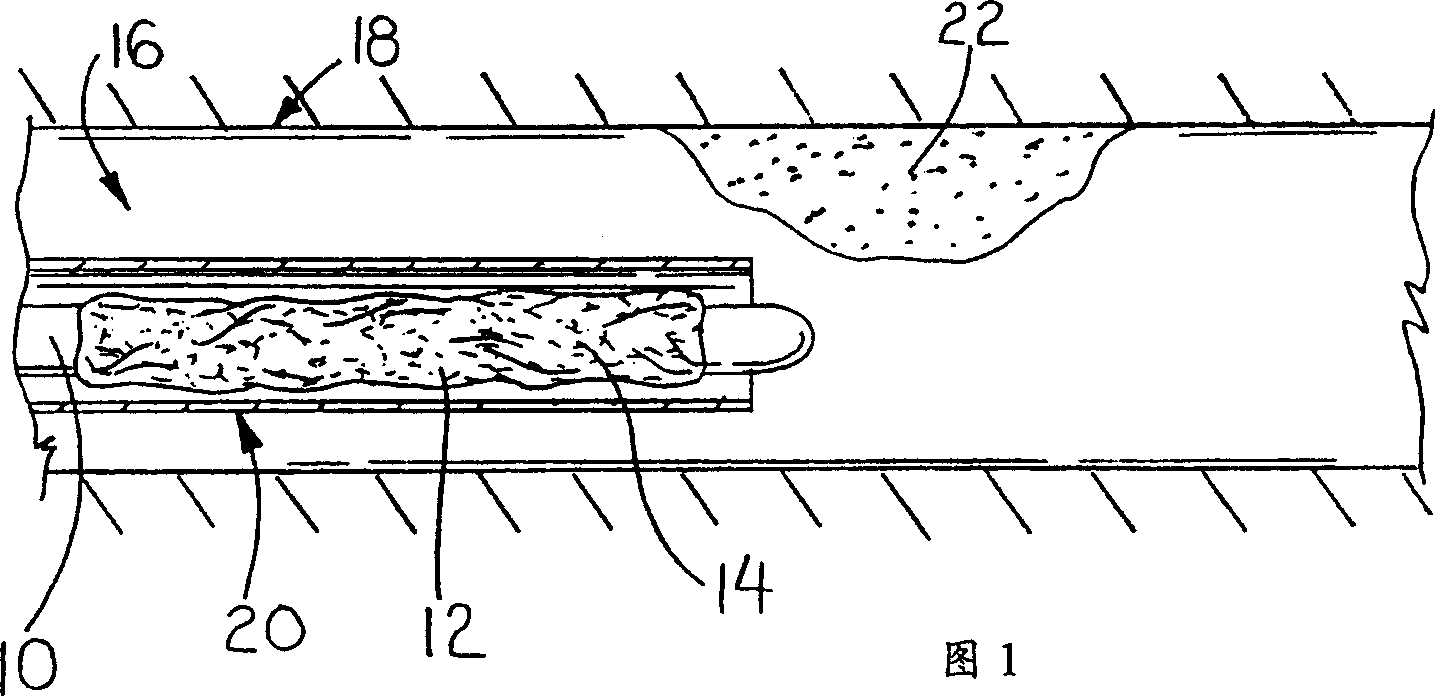
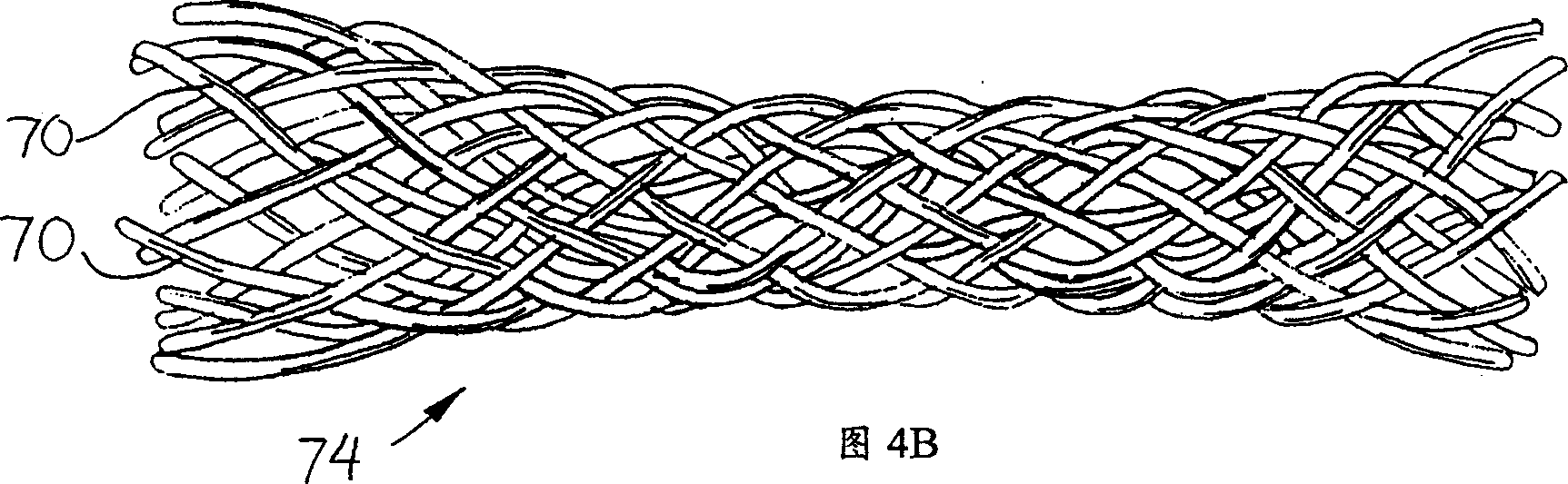
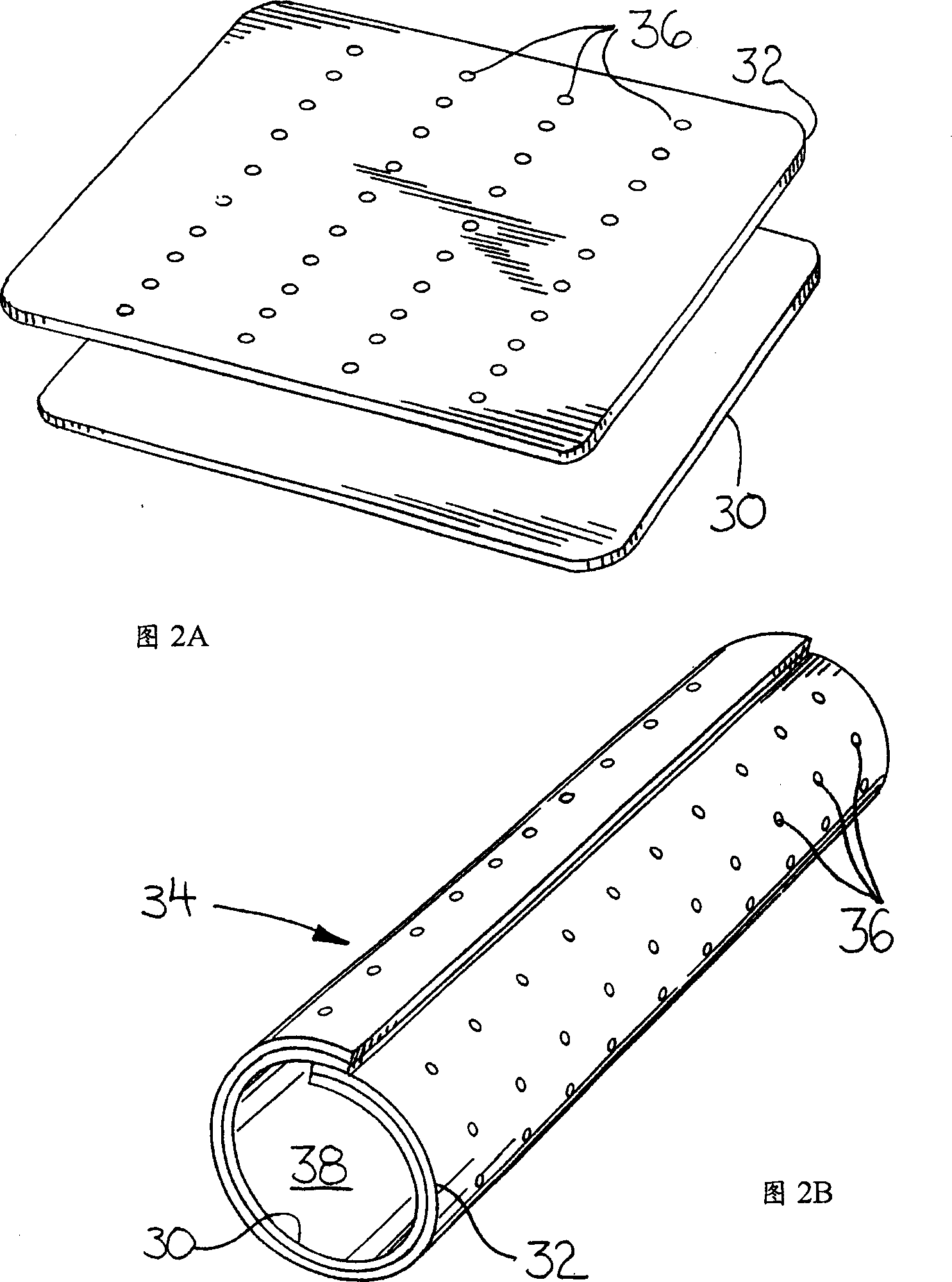
Use of VEGF-C or VEGF-D gene or protein to prevent resitenosis
A VEGF-C, VEGF-D technology, applied in the field of cardiovascular medicine, can solve problems such as arterial intimal hyperplasia and deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Application of adenovirus-mediated VEGF-C gene transfer to prevent restenosis
[0087] The following experiments were carried out in vivo in a rabbit model of restenosis to demonstrate the efficacy of adenovirus-mediated intravascular VEGF-C gene transfer in preventing restenosis after angioplasty.
[0088] AMaterials and methods
[0089] 1. Adenovirus Construction
[0090] An adenoviral plasmid comprising a cDNA encoding the entire human pre-VEGF-C open reading frame operably linked to the cytomegalovirus (CMV) promoter and human growth hormone polyadenylation signal sequence was constructed as follows. A DNA fragment containing the CMV promoter sequence was prepared by cutting the pcDNA3.1+ vector (Invitrogen) with SalI and filling in the 5' overhang with Klenow enzyme. The CMV promoter (5431-911 nucleotides) was digested and isolated from the vector with HindIII. With HindIII and XhoI, the full-length human VEGF-C cDNA (and less than 50 bases of noncoding sequence a...
Embodiment 2
[0104] Comparative examples confirm that the anti-restenotic effect of VEGF-C is stronger than that of VEGF
[0105] The experiments described below demonstrate the preventive effect of adenovirus-mediated intravascular VEGF and VEGF-C gene transfer on restenosis after angioplasty, and demonstrate that VEGF-C exhibits stronger therapeutic efficacy compared to VEGF.
[0106] AMaterials and methods
[0107] 1. Adenovirus Construction
[0108] Apply the same promoter as VEGF-C, and use the method similar to Example 1 to construct VEGF adenovirus (mouse VEGF-A 164 , SEQ IN NO: 18). Production of VEGF-A in 293T cells 164 Constructs, concentrated essentially as described in Example 1, were determined to be free of helper virus, lipopolysaccharide, and bacterial contaminants.
[0109] 2. Animal models
[0110] Sixty-three New Zealand white rabbits were divided into two groups, the first group was fed with 0.25% cholesterol diet for 2 weeks, and underwent aortic dissection before...
Embodiment 3
[0122] Expression of transfected VEGFs on blood vessel wall
[0123] Utilizing the aortic fragments of the same experimental animal as in Example 2, lacZ, VEGF-C and VEGF (mouse VEGF-A) in the aortic tissue after gene transfer were analyzed. 164 ) mRNA expression. Total RNA was extracted from transfected aortic fragments using Trizol reagent (Gibco-BRL), and 2 μg was used for cDNA synthesis. To distinguish endogenous genes from transduced genes, primers for lacZ, VEGF-C and VEGF were designed by selecting 5' primers from the CMV promoter and 3' primers from the coding region.
[0124] In the amplification of lacZ, the primers are: 5' primer 5'-TTGGAGGCCTAGGCTTTT GC-3' (SEQ ID NO: 5), 3' primer 5'-ATACTGTCGTCGTCCCCTCA-3' (SEQ ID NO: 6). The first PCR cycle was an initial incubation at 96°C for 4 minutes, followed by 3 minutes at 80°C, during which time DNA polymerase was added. This was followed by 30 cycles, each cycle consisting of 94°C for 45 seconds, 58°C for 45 seconds,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com