Peparation of human recombined blood-vessel generation element and skin whitening product
An angiogenin, skin whitening technology, applied in the direction of angiogenin, angiogenesis factors, animal/human protein, etc., can solve the problem that there are no cosmetics containing human angiogenin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Gene expression of human angiopoietin
[0051] In this example, according to the amino acid sequence of human angiogenin, its coding nucleotide sequence was synthesized by chemical synthesis. Considering the specificity of strains used in genetic engineering, codons favored by Escherichia coli B21 cells were used in the process of designing coding nucleotides. The obtained human angiopoietin coding sequence is shown in SEQ ID NO: 1 and Figure 1.
[0052] Figure 1 lists the nucleotide sequences encoding human angiogenin used in the present invention. The nucleotide sequence used in the present invention is different from the human natural gene sequence encoding angiogenin (SEQ ID NO: 3 and Fig. 1). However, the amino acid sequences of their encoded proteins (SEQ ID NO: 2 and Fig. 1) are identical. Using the nucleotide sequence of angiogenin designed in the present invention, the highly expressed angiogenin can be obtained in bacteria by means of genetic engineering. ...
Embodiment 2
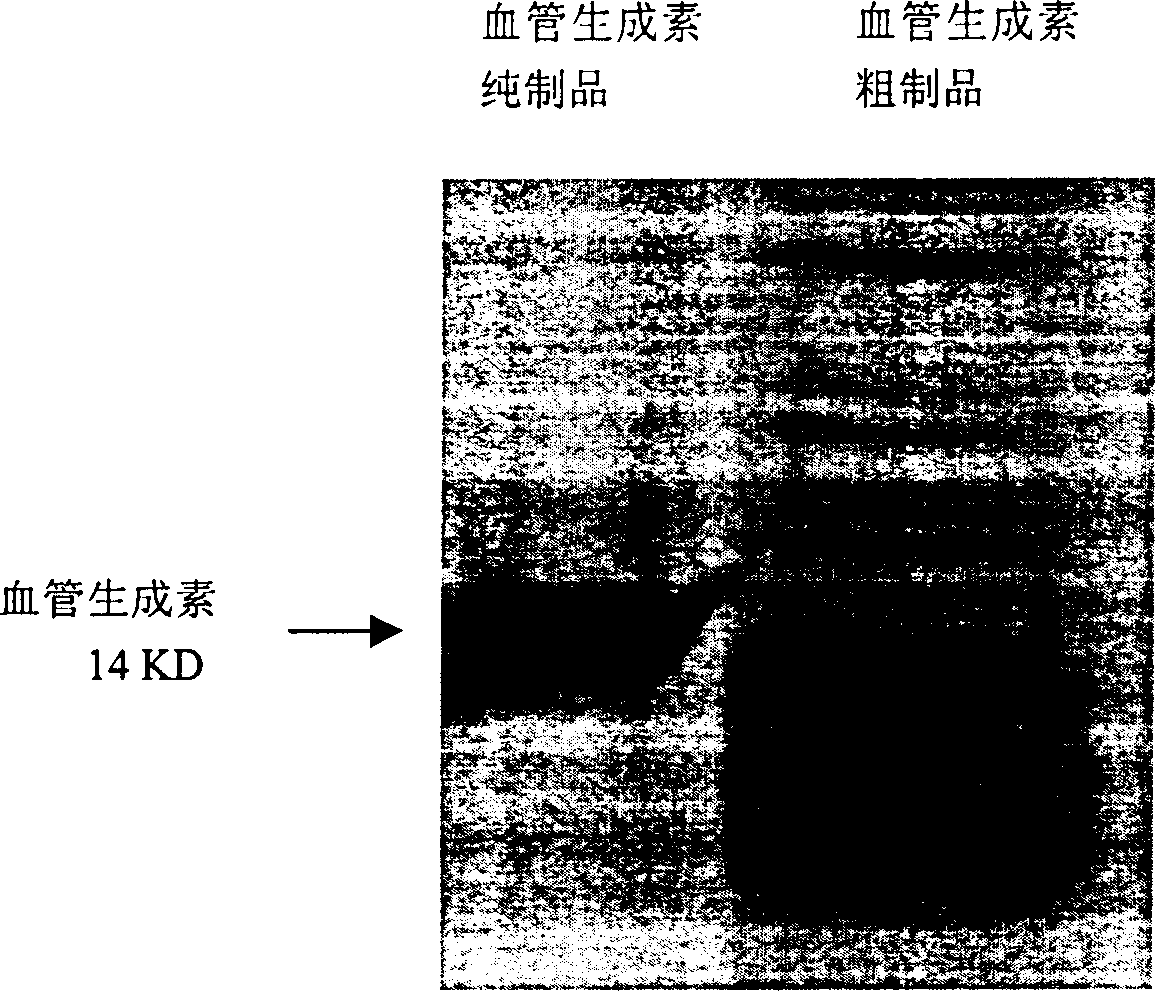
[0063] Biochemical Purification of Human Angiopoietin
[0064] In the embodiment, the angiogenin expressed by the genetic engineering technique in the embodiment 1 was purified by a biochemical method. This purification method, with proper modification, is also suitable for extracting angiogenin from other materials including human plasma, milk and tumor cell secretion.
[0065] (1) The inclusion body containing angiopoietin prepared by the above expression was dissolved with 7 equivalents of guanidine hydrochloride, and after further sonication, 2-mercaptoethanol (0.7% final concentration) was added and incubated at 37°C for 3 hours to make the disulfide bond completely Open.
[0066](2) Slowly add the guanidine hydrochloride solution of angiopoietin with the above-mentioned peptide chain fully extended, dilute it and add it to 100 times the volume of the peptide chain refolding solution (50mM Tris-HCl, 100mM NaCl, pH8.5), statically at 4°C Leave to insulate for 8 hours, th...
Embodiment 3
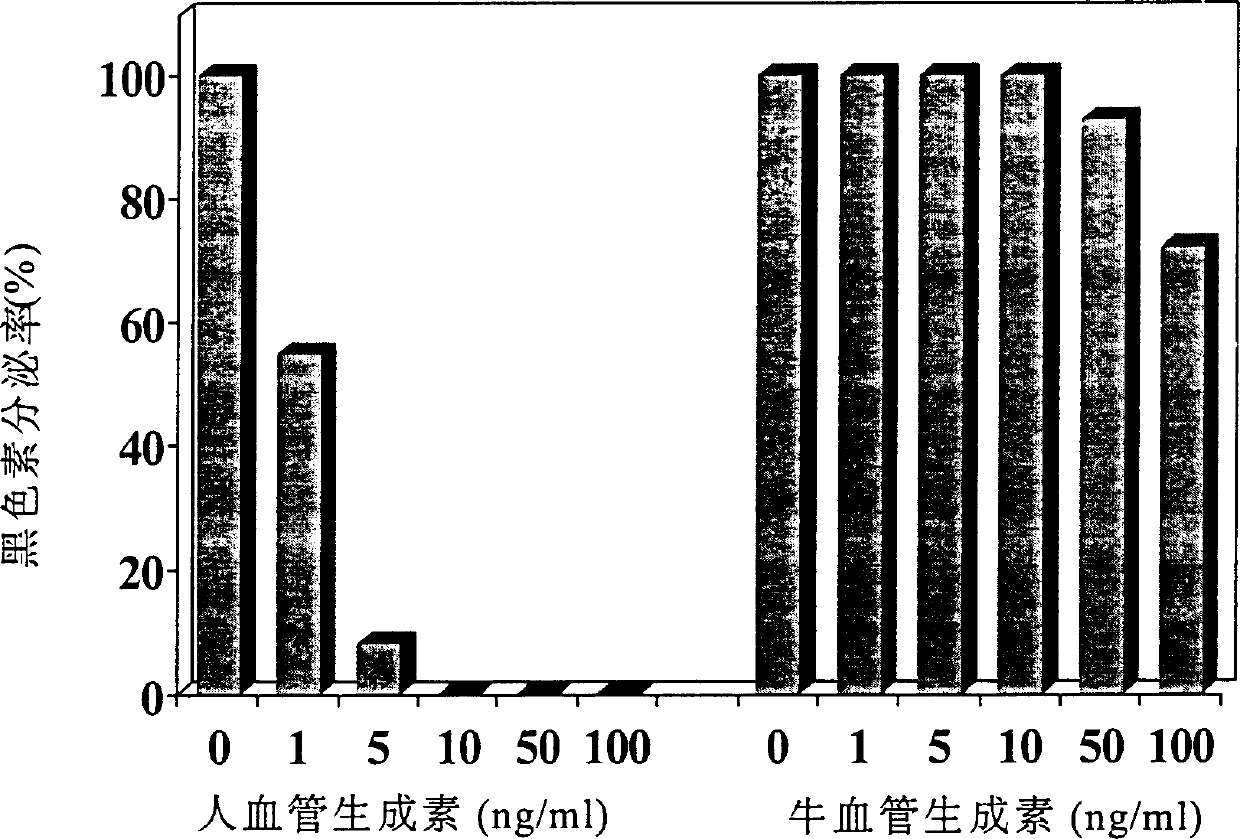
[0077] Angiogenin activity assay
[0078] In this example, the inhibitory effect of the angiogenin obtained in Example 2 on melanin secretion by normal human melanocytes was tested.
[0079] (1) Normal human melanocytes were purchased from Clonetics Company in the United States and cultured in a special medium provided by the company. The culture conditions are constant temperature and humidity at 37°C and 5% carbon dioxide gas phase. The medium was changed every two days, and the culture medium was subcultured at a ratio of 1:3.
[0080] (2) The adherent melanocytes were digested with trypsin-EDTA, the cells were collected by centrifugation, suspended in complete medium, and seeded in a 96-well cell culture plate at a density of 30,000 cells per well.
[0081] (3) Add different concentrations of samples to be tested (human angiogenin or bovine angiogenin at different concentrations) to each well, and incubate at 37° C. under the above culture conditions.
[0082] (4) Measu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com