Hyaluronic acid oligosaccharide fractions and drugs containing the same
A technology of hyaluronic acid and oligosaccharides, applied in the direction of medical preparations containing active ingredients, sugar derivatives, sugar derivatives, etc., can solve problems such as no description or hint
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0207] Preparation and physicochemical properties of oligosaccharides of the present invention and fractions of the present invention
[0208] 1. The preparation of oligosaccharide of the present invention and fraction of the present invention
[0209] The oligosaccharide of the present invention and the fraction of the present invention were prepared using the sodium salt of HA isolated and purified from cockscomb as a raw material by the following method. In addition, the sodium salt of HA as a raw material showed a single band by electrophoresis using a cellulose acetate membrane (electrophoresis buffer: pyridine-formic acid buffer, current: 15 mA, electrophoresis time: 30 minutes), and HA was not detected Other mucopolysaccharides (chondroitin, 4-chondroitin sulfate, 6-chondroitin sulfate, chondroitin sulfate E, chondroitin sulfate D, heparin, heparan sulfate, dermatan sulfate). (Preparation Example 1) Decomposition by Hyaluronidase
preparation example 1
[0209] The oligosaccharide of the present invention and the fraction of the present invention were prepared using the sodium salt of HA isolated and purified from cockscomb as a raw material by the following method. In addition, the sodium salt of HA as a raw material showed a single band by electrophoresis using a cellulose acetate membrane (electrophoresis buffer: pyridine-formic acid buffer, current: 15 mA, electrophoresis time: 30 minutes), and HA was not detected Other mucopolysaccharides (chondroitin, 4-chondroitin sulfate, 6-chondroitin sulfate, chondroitin sulfate E, chondroitin sulfate D, heparin, heparan sulfate, dermatan sulfate). (Preparation Example 1) Decomposition by Hyaluronidase
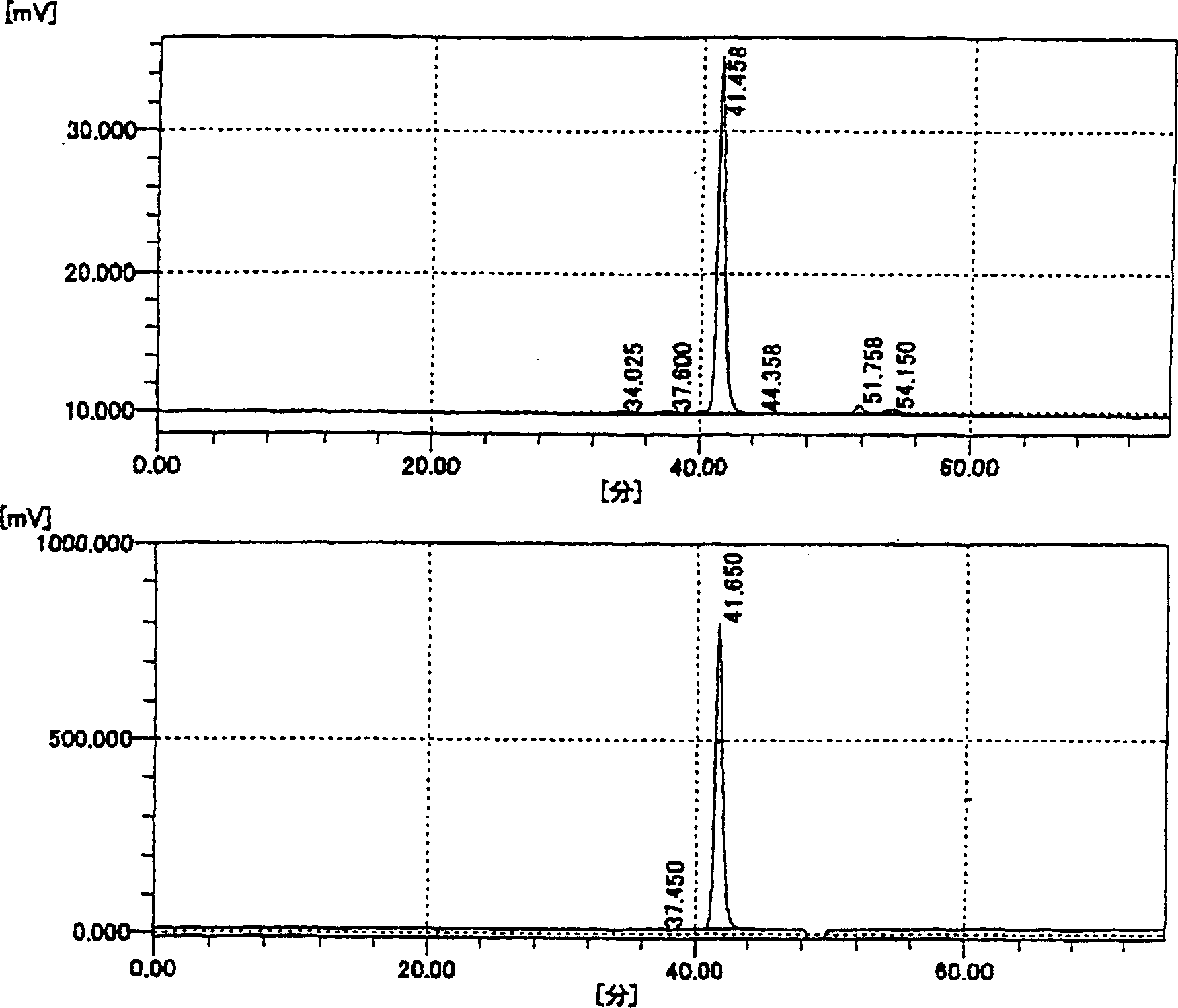
[0210] Dissolve 25 g of sodium salt of HA in 1.IL of 0.1 M phosphate buffer (pH 5.3) containing 0.15 M NaCl. To the obtained solution, 200 mg of hyaluronidase (5.342 units / mg; manufactured by Seikagaku Kogyo Co., Ltd.) derived from bovine testis was added and reacted at 37° C. for 9...
preparation example 2
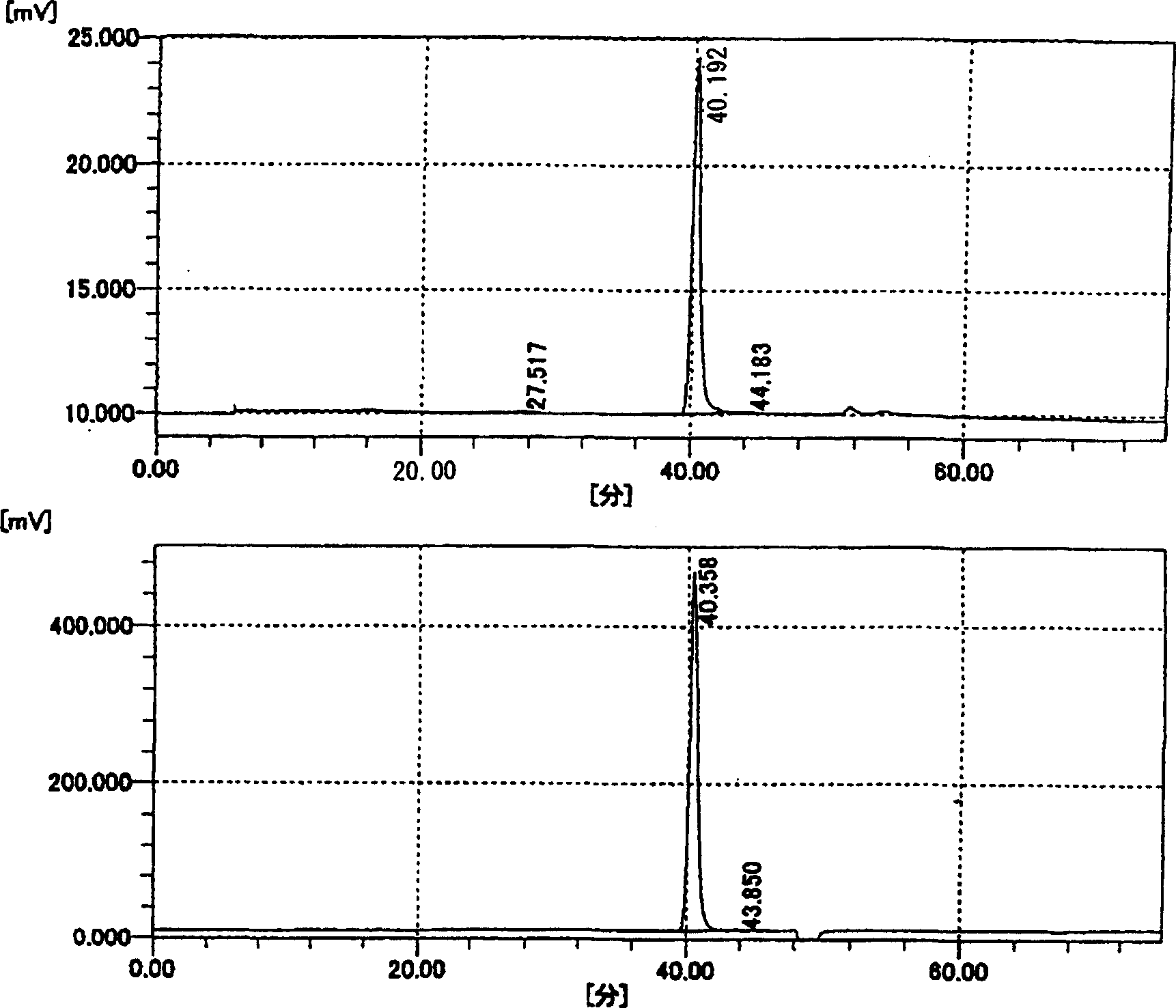
[0213] HA4(330mg), HA6(1210mg), HA8(305mg), HA10(1625mg), HA12(685mg), HA14(620mg), HA16(430mg), HA18(210mg), HA20(202mg), HA22(819mg), HA24(197mg), HA26(187mg), HA28(159mg), HA30(137mg), HA32(122mg), HA34(102mg), HA36(91mg), HA38(89mg), HA40(65mg), HA42(76mg), HA44 (61 mg), HA46 (58 mg), HA48 (46 mg), HA50 (48 mg), HA52 (21 mg). (Preparation Example 2) Decomposition of ACI by Chondroitinase
[0214] Dissolve 8 g of sodium salt of HA in 500 ml of 0.1 M acetic acid buffer (pH6.0) containing 0.1% bovine serum albumin.
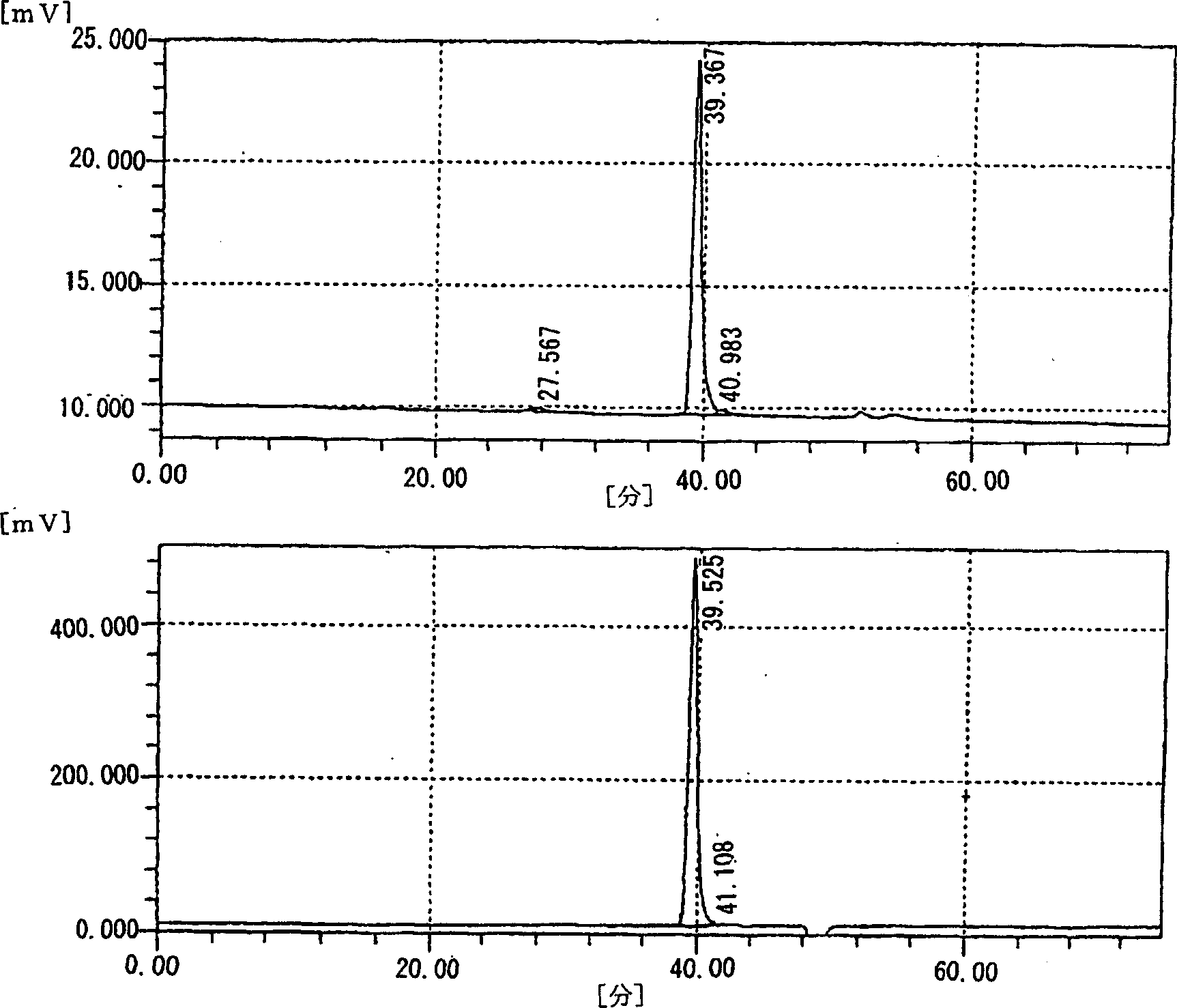
[0215] To the obtained solution, 32 units of chondroitinase ACI (manufactured by Seikagaku Kogyo Co., Ltd.) was added and reacted at 37° C. for 6 hours.
[0216] After the reaction, the supernatant was collected by centrifugation at 10,000 rpm for 30 minutes. The recovered supernatant was loaded onto a Dowex 1×2 (100-200 mesh) (manufactured by Dow chemical) ion-exchange column (φ4.5×123 cm), and eluted by a linear concentration gradient (0.01M-0.50M) of NaCl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com