Twist structured p-phenylene ethylene luminous polymer and use thereof
A technology of p-phenylene vinylene and light-emitting polymers, which is applied in the field of conjugated light-emitting polymers, can solve problems such as low carrier mobility, difficulty in blue light, and limited range of blue shift, so as to improve blue light-emitting performance, Effect of improving quantum efficiency and inhibiting association
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
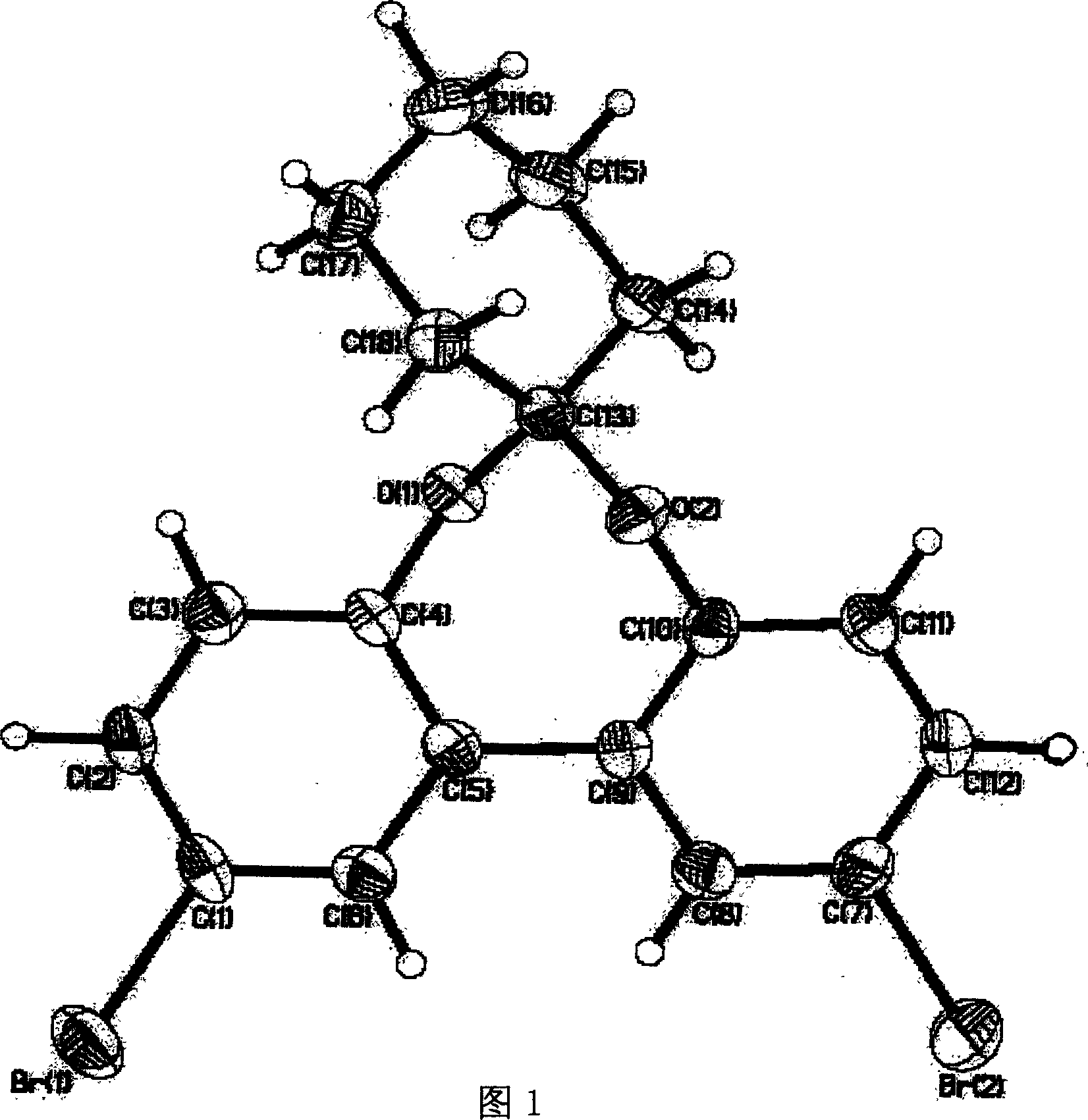
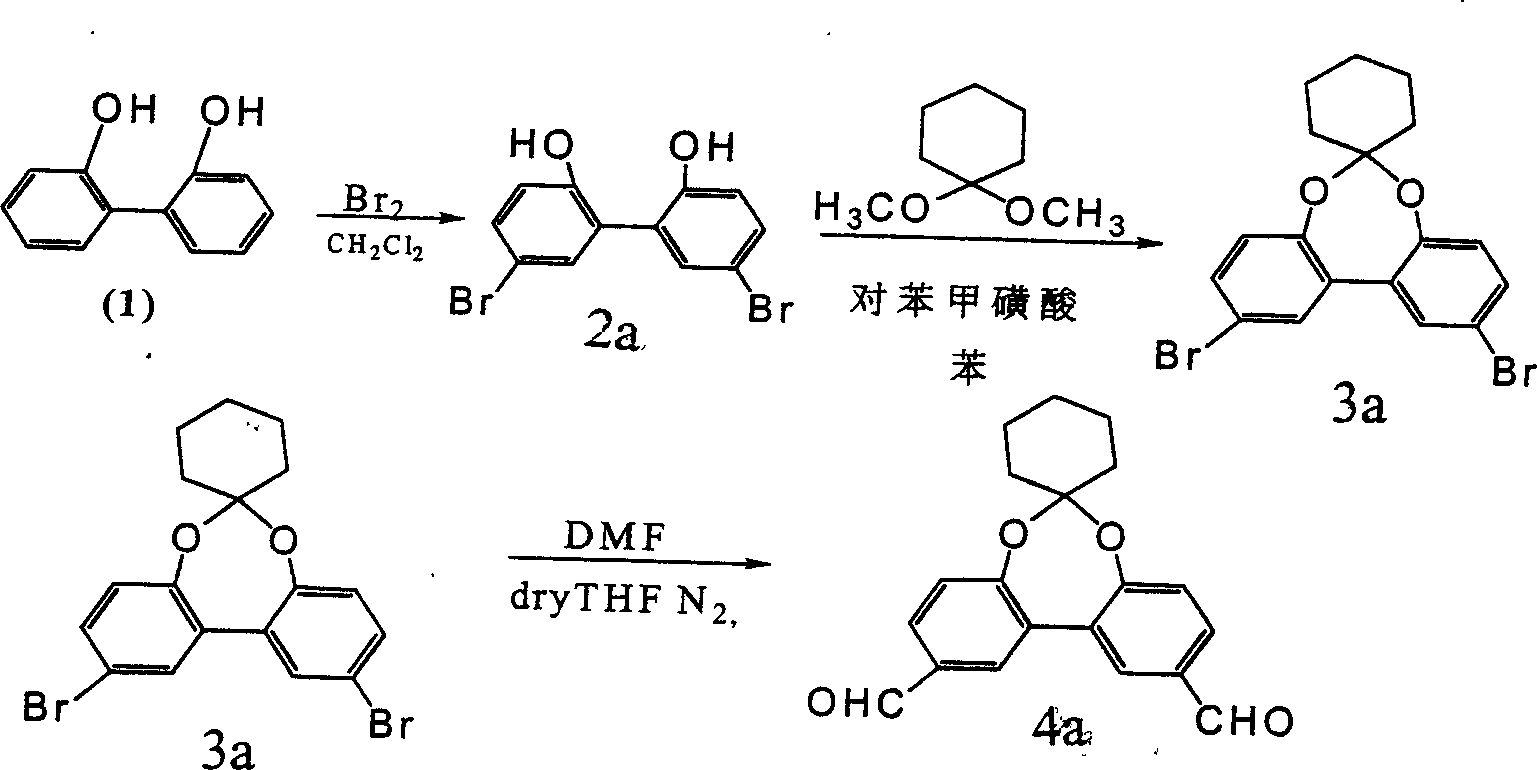
[0046]Embodiment 1 6-cyclohexyl-2, the synthesis of 10-dialdehyde-bisphenyl [d, f] [1,3] bisoxazepine (synthetic route sees figure 2 ) Dissolve compound 1 (2,2'-dihydroxybiphenyl) in dichloromethane, stir vigorously at room temperature, add equimolar bromine, stop the reaction when precipitation occurs, and obtain compound 2a, 5, 5 '-Dibromo-2,2'-dihydroxybiphenyl,
[0047] Dissolve compound 2a (5,5'-dibromo-2,2'-dihydroxybiphenyl) in benzene, add p-toluenesulfonic acid and equimolar 2,2'-dimethoxycyclohexane, heat, Remove the components at 57-59°C, stop the reaction, and neutralize with sodium methoxide. Extracted with ether, dried over anhydrous sodium sulfate, removed the solvent, and recrystallized from ethanol to obtain compound 3a (6,6-cyclohexyl-2,10-dibromo-bisphenyl[d,f][1,3]bisoxazene ),
[0048] Compound 3a (6,6-cyclohexyl-2,10-dibromo-bisbenzo[d,f][1,3]bisoxazepine) was added to 20 ml of dry tetrahydrofuran under nitrogen protection. Add n-BuLi (n-butyllithium)...
Embodiment 2
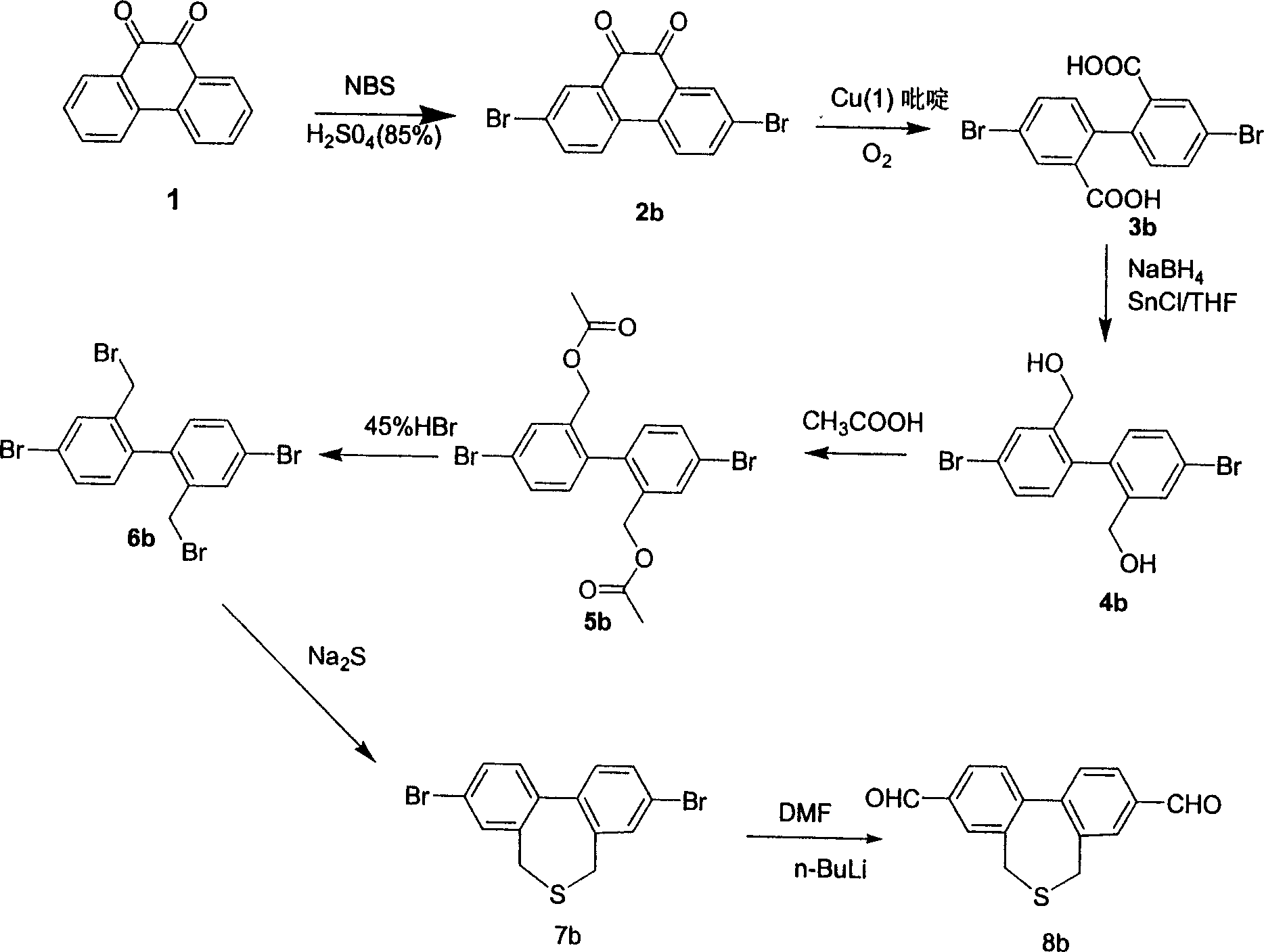
[0049] Example 2 Twisted monomer 2', 3 "-dialdehyde-5, the synthetic route of 7-diphenylthiatable table (route see image 3 ) Add NBS (N-bromosuccinimide) to 85% sulfuric acid solution of compound 1 and stir vigorously for 1 hour, add water and stir, extract with acetone, and recrystallize with DMSO (dimethyl sulfoxide) to obtain compound 2b;
[0050] In the presence of oxygen, the mixture of compound 2b and ketone chloride was dissolved in pyridine and stirred for 6 hours, extracted with ether, dried and recrystallized to obtain compound 3b,
[0051] NaBH 4 Slowly add to THF (tetrahydrofuran) and cool to -78°C, add SnCl under nitrogen 4 , vigorously stirred for 30min, and the temperature was raised to 0°C, the THF solution of compound 3b was added dropwise to the above solution once, slowly brought to room temperature and vigorously stirred for 48h, then poured into ice water, extracted with ether, dried, and column chromatographed to obtain the compound 4b; After acidific...
Embodiment 3
[0054] Example 3 Wide Bandgap Blue Light Emitting PPV-like Polymers
[0055] Synthesis of SCHDDBDOP-PPV (poly(2,10-(6-cyclohexyl-bisphenyl [d, f] [1,3] dioxazepine)-1,4 phenylene vinylene)) (route see Figure 4 )
[0056] The dried drug 2,5-dibromomethylbenzene and triphenylphosphine were distilled in DMF (N,N-dimethylformamide) for 14 hours at a molar ratio of 1:2.02, and the phosphine was filtered out after cooling. The salt precipitated, washed with p-xylene and dried. Under nitrogen protection conditions, in a fully dry reaction device, add phosphine salt, potassium tert-butoxide, and drug 4a in a molar ratio of 2:3:2.02, react in THF at 0°C for 6 hours, wash with methanol four times, Dry to obtain a polymer.
[0057] The optical properties of the above-mentioned blue light-emitting PPV polymers:
[0058] The absorption and emission spectra of the blue light-emitting polymer SCHDDDOP-PPV in solution and film (excitation light source is 350nm) such as Figure 5 As show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com