Prepn process of submicron level positive pole material for lithium ion cell
A technology for lithium ion batteries and cathode materials, which is applied in the field of liquid phase synthesis, can solve the problems of large ion arrangement disorder, wide particle size distribution range, poor chemical uniformity, etc., and achieves excellent electrochemical performance, shortened synthesis cycle, and processing time. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
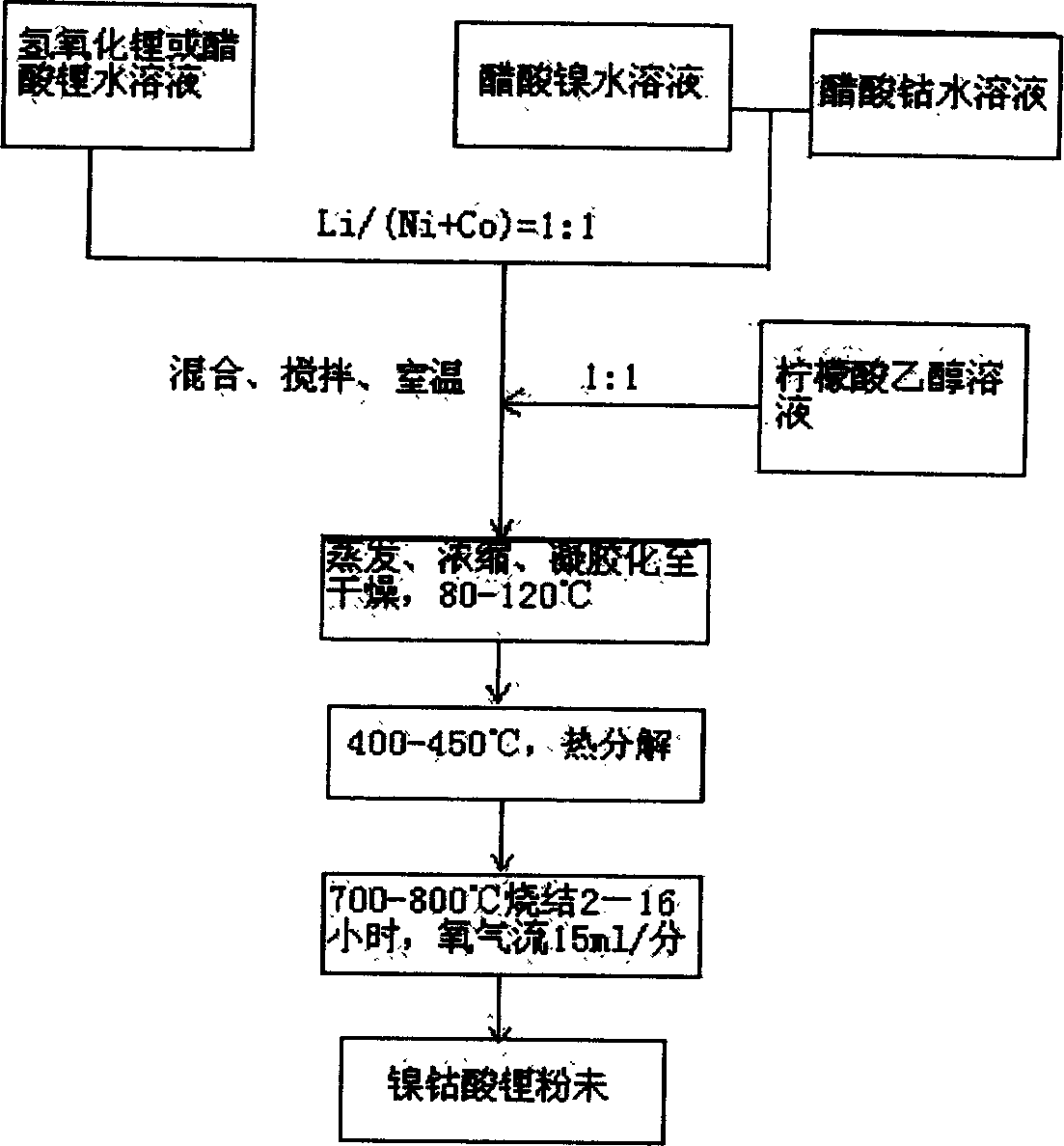
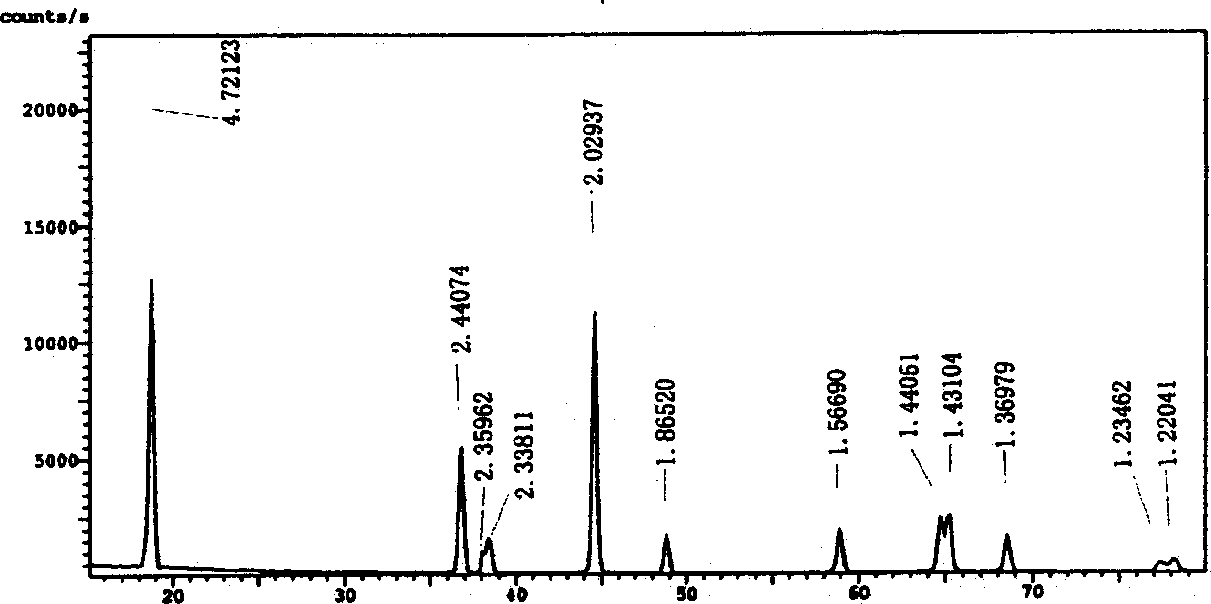
[0030] First weigh 0.115mol of LiOH·H 2 O was dissolved in 30ml of distilled water to form a solution, and another 0.08mol of nickel acetate and 0.02mol of cobalt acetate were dissolved in 150ml of distilled water to form a transparent deep purple solution; the two aqueous solutions were mixed and stirred to form a deep purple suspension. Then gradually add 0.1mol citric acid solution dissolved in 90ml ethanol to it, stir continuously until a clear and uniform solution is formed, heat to 80°C and stir to evaporate the solvent, concentrate for about 3 hours to form a gel, and then dry it in an oven to obtain the precursor body dry gel. Put the dry gel in a muffle furnace at 400°C for 8 hours to decompose the organic matter, take it out and grind it into a powder (brown); the powder is then sintered in a tube furnace at a rate of 3°C / min to 800°C 2 Hours later, lower the temperature to 700°C and roast for 12 hours. During the sintering process, the oxygen flow is 15ml / min. Fina...
Embodiment 2
[0032] Accurately weigh 0.115mol of LiOH·H 2 O, 0.07mol of nickel acetate and 0.03mol of cobalt acetate were dissolved in distilled water to form an aqueous solution, mixed and stirred to form a deep purple suspension, and then gradually added 0.1mol of citric acid solution dissolved in 90ml of ethanol, heated at 100 ° C , evaporate, concentrate to form a gel, and dry; decompose the organic matter in the air at 450°C, and then sinter at 800°C for 4 hours, then cool down to 720°C and continue sintering for 12 hours to obtain the positive electrode material LiNi 0.7 co 0.3 o 2 Powder is not.
[0033] The electrochemical performance test was carried out according to the method of Example 1. The test results at room temperature are as follows Figure 5 As shown, the initial reversible capacity is up to 174mAh / g, the first charge-discharge coulombic efficiency is 89%, and after 30 cycles, the reversible capacity is still 156mAh / g, indicating that the material has excellent elec...
Embodiment 3
[0035] Accurately weigh 0.108mol of Li(CH 3 COO), 0.08mol of nickel acetate and 0.02mol of cobalt acetate were dissolved in distilled water to form an aqueous solution, mixed and stirred to form a dark purple suspension, and then gradually added 0.1mol of citric acid solution dissolved in 90ml of ethanol, at 100 ° C Heating, evaporating, concentrating to form a gel, and drying; decompose the organic matter in the air at 450°C, and then sinter at 800°C for 12 hours to obtain the positive electrode material LiNi 0.8 co 0.2 o 2 Powder is not. Its electrochemical performance test at room temperature has an initial reversible capacity of up to 170.2mAh / g, and a coulombic efficiency of 87.1% for the first charge and discharge. After 30 cycles, the reversible capacity is still 146mAh / g, indicating that the material has excellent electrochemical properties.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com