Process for preparing size-controllable nano-silicon dioxide
A nano-silicon dioxide and size technology, applied in the preparation of silicon dioxide, silicon oxide, oxide/hydroxide, etc., can solve the problems of poor cooling effect, sintering into large particles, and difficult size, so as to improve the cooling effect. Efficiency and effect of preventing particle size increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
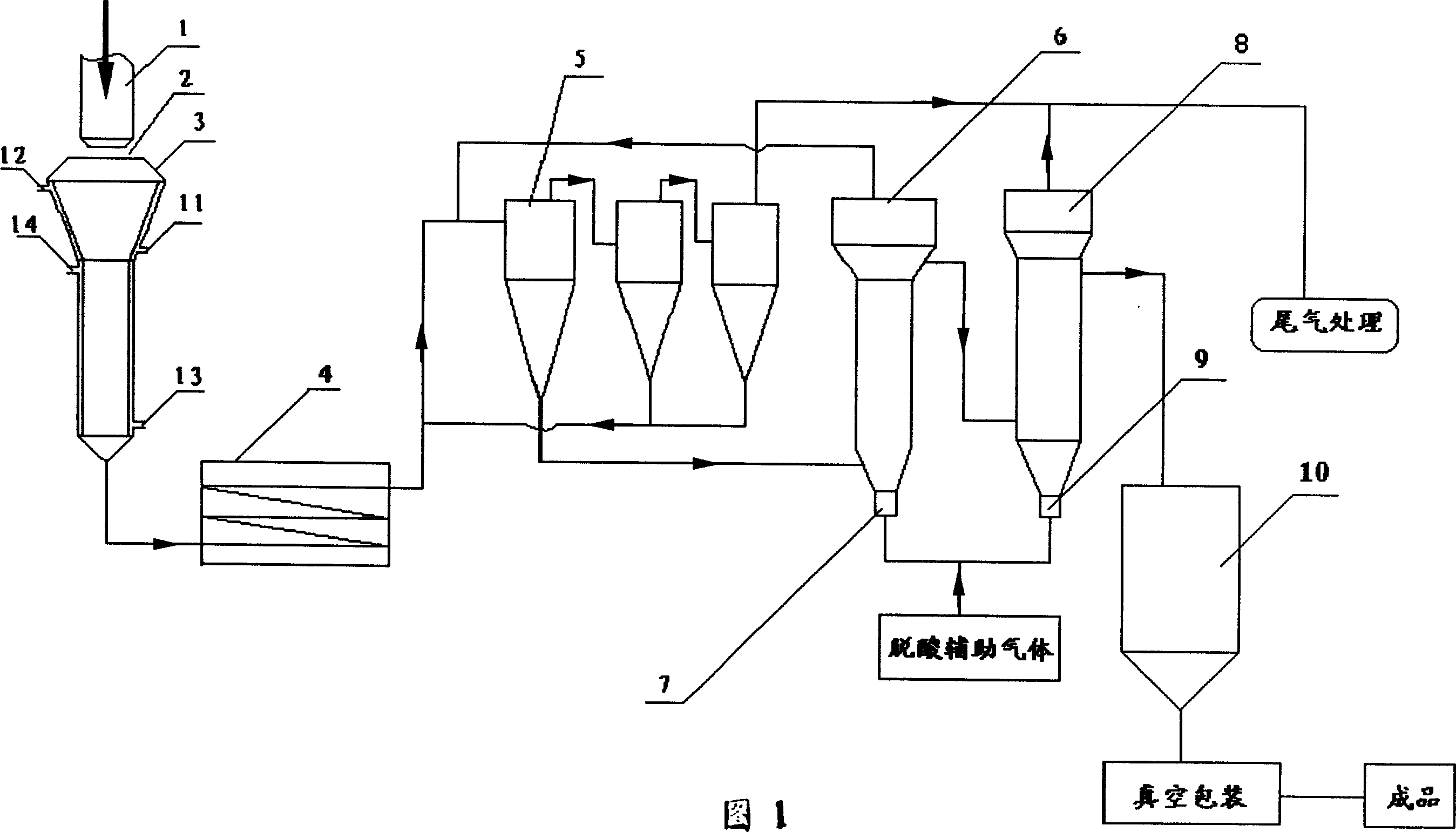
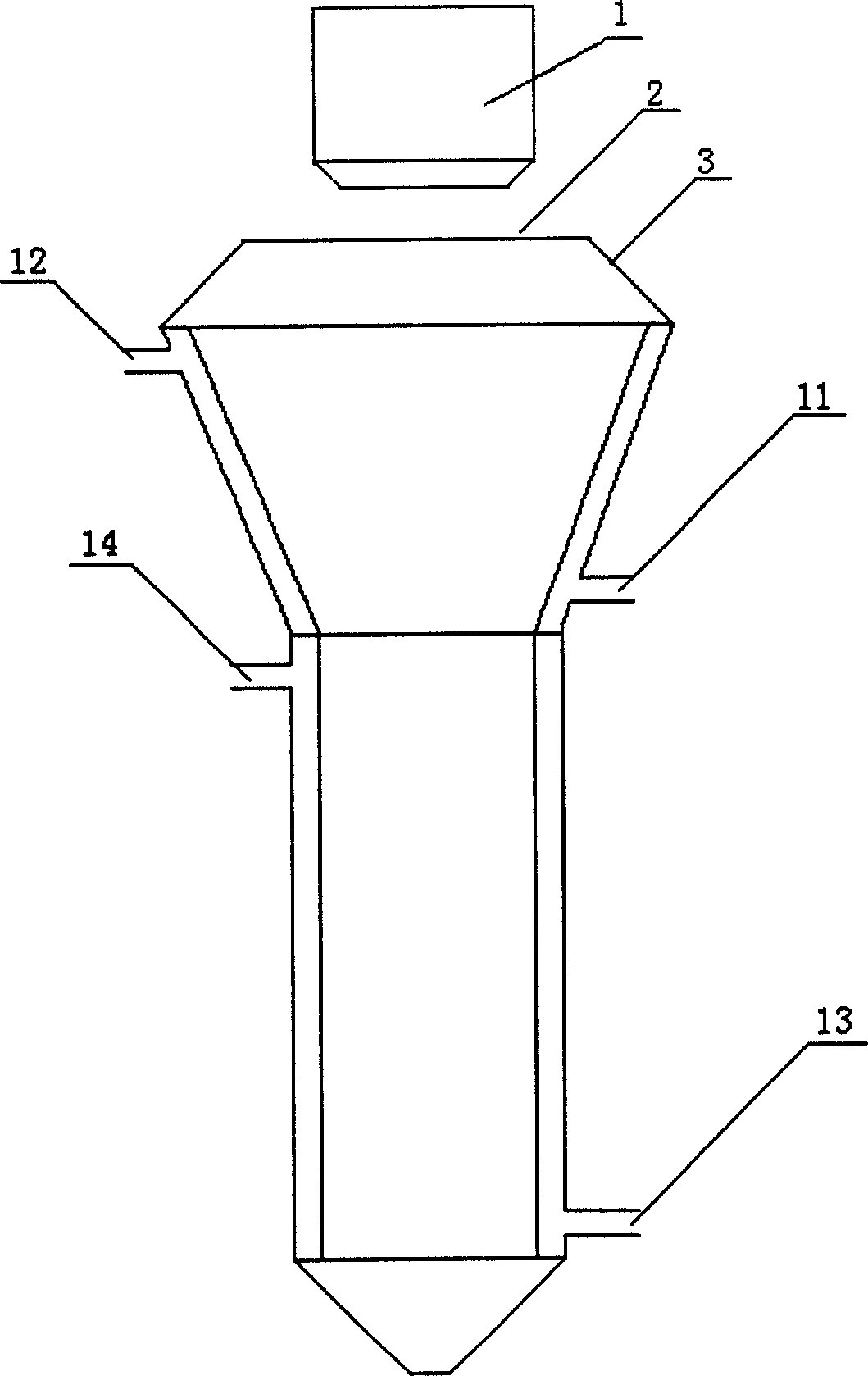
[0022] Methyltrichlorosilane at 7.5m 3 / h, hydrogen at 12m 3 / h, air at 105m 3 The supply of / h is continuously delivered to the premixer for premixing (the above volume is the standard volume), and then enters the nozzle of the combustion furnace after preheating to 120°C. The specific process is shown in Figure 1. The temperature in the reaction chamber is 720°C. The temperature in the primary deacidification furnace is 600°C, the temperature in the secondary deacidification furnace is 550°C, the auxiliary gas for deacidification is water vapor and nitrogen, and the temperature is 230°C. The quality indicators of the prepared silica are as follows:
[0023] Silica content (%) (hydrofluoric acid method) 99.85
[0024] Primary particle average particle size (nm) (electron microscope method) 30
[0025] Specific surface area (m2 / g) (BET method) 153
[0026] PH value (4% water suspension) 3.95
[0027] Carbon content (%) 0.01
Embodiment 2
[0029] The methyl trichlorosilane of embodiment 1 is replaced with silicon tetrachloride, and silicon tetrachloride is 7m 3 / h, hydrogen at 15m 3 / h, air at 130m 3 The supply of / h is supplied, the process is the same as in Example 1, and the temperature in the reaction chamber is 640° C., and the others are the same as in Example 1. The quality index of the prepared silica is as follows:
[0030] Silica content (%) (hydrofluoric acid method) 99.85
[0031] Primary particle average particle size (nm) (electron microscopy) 12
[0032] Specific surface area (m 2 / g) (BET method) 283
[0033] PH value (4% water suspension) 4.05
[0034] Carbon content (%) Not determined
Embodiment 3
[0036] The methyltrichlorosilane in Example 1 is replaced with 60% methyltrichlorosilane and 40% silicon tetrachloride, and the supply is 8.5m 3 / h, hydrogen is 16m 3 / h, the air is 125m 3 / h, the temperature in the reaction chamber is 690°C, and other process parameters are the same as in Example 1, and the quality index of the prepared silicon dioxide is as follows:
[0037] Silica content (%) (hydrofluoric acid method) 99.82
[0038] Primary particle average particle size (nm) (electron microscopy) 15
[0039] Specific surface area (m 2 / g) (BET method) 208
[0040] PH value (4% water suspension) 4.23
[0041] Carbon content (%) 0.01
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com