Preparation of oxidized inserting layer of laminated lithium manganate as lithium ion battery anode
A positive electrode material layer, lithium-ion battery technology, applied in the direction of electrode manufacturing, lithium batteries, battery electrodes, etc., can solve the problems of reaction vessel volume limitation, high price, harsh reaction conditions, etc., achieve uniform particle size controllable, low production cost The effect of low cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
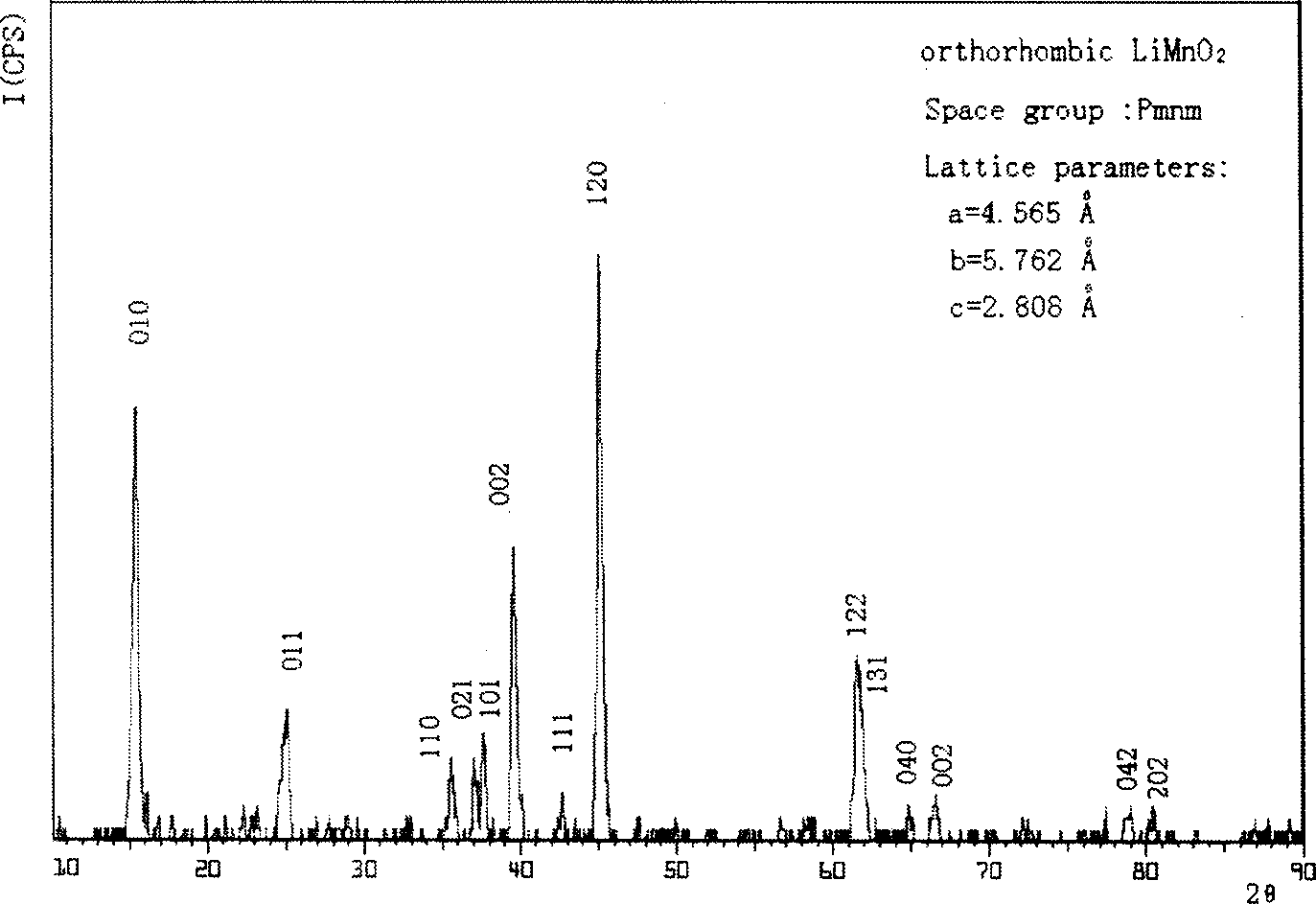
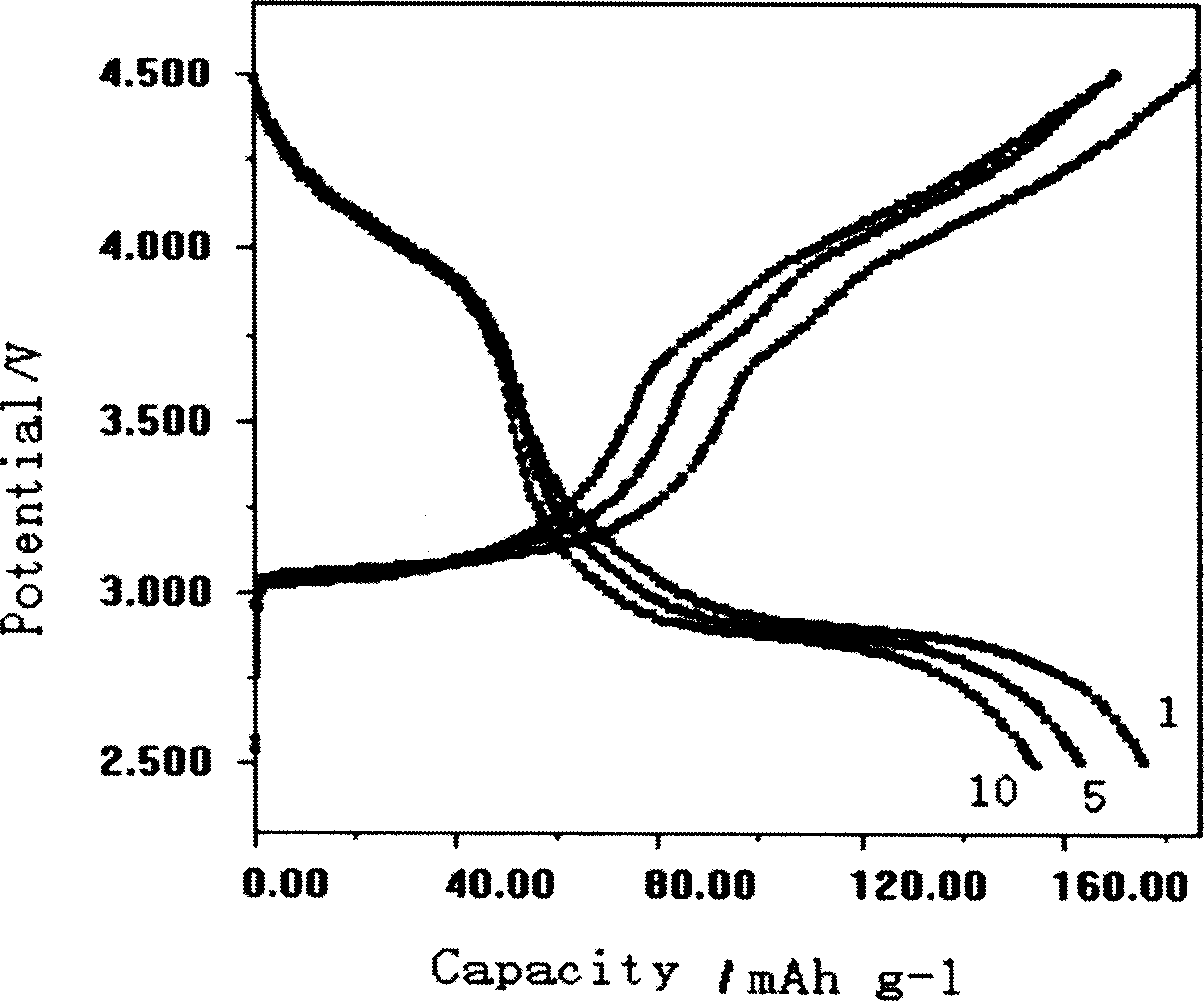
[0029] Weigh MnSO 4 ·H 2 O 16.90g (0.10mol), NaOH 8.00g (0.20mol), in N 2 Dissolve them in 90 mL of boiled deionized water under protection; drop the NaOH solution into the MnSO 4 In solution, the obtained Mn(OH) 2 The precipitate was aged in a water bath at 60°C for 10 hours; weigh LiOH·H 2 O solid powder 20.98g (0.5mol), added to the above Mn (OH) 2 in the suspension and stir to dissolve; weigh the oxidizing agent (NH 4 ) 2 S 2 o 8 17.10g (0.075mol), dissolved in 70mL of boiled deionized water, added dropwise to the reaction system at a uniform rate within 2h, and continued to react in a water bath at 80°C for 10h; filter the product with a glass sand funnel, and then Wash and filter with 180mL deionized water for 3 times, and then vacuum dry at 120°C for 8 hours to obtain the product of the present invention. ICP and XRD analysis showed that the product composition was Li 0.83 MnO 2 , is an orthorhombic layered lithium manganate structure ( figure 1 ), the elec...
Embodiment 2
[0031] Weigh MnSO 4 ·H 2 O 16.90g (0.10mol), KOH 12.34g (0.22mol), in N 2 Dissolve in 90mL boiled deionized water under protection; drop KOH solution into MnSO4 under rapid stirring 4 In solution, the obtained Mn(OH) 2 The precipitate was aged in a water bath at 80°C for 10 hours; weigh LiOH·H 2 O solid powder 41.96g (1.0mol), added to the above Mn(OH) 2 in the suspension and stir to dissolve; weigh K 2 S 2 o 8 Dissolve 16.20g (0.06mol) of oxidizing agent in 70mL of boiled deionized water, drop it into the reaction system at a uniform rate within 2h, and continue the reaction in a water bath at 80°C for 10h; filter the product with a glass sand funnel and then Wash and filter with 180mL deionized water for 3 times, and then vacuum dry at 120°C for 12h to obtain the product of the present invention. ICP and XRD analysis showed that the product composition was Li 0.91 MnO 2 , which belongs to the orthorhombic layered lithium manganese oxide structure, and the electroch...
Embodiment 3
[0033] Weigh MnSO 4 ·H 2 O 16.90g (0.10mol), NaOH 8.80g (0.22mol), in N 2 Dissolve them in 90 mL of boiled deionized water under protection; drop the NaOH solution into the MnSO 4 In solution, the obtained Mn(OH) 2 The precipitate was aged in a water bath at 80°C for 10 hours; weigh LiOH·H 2 O solid powder 41.96g (1.0mol), added to the above Mn(OH) 2 in the suspension and stir to dissolve; weigh (NH 4 ) 2 S 2 o 8 17.10 g (0.075 mol) of oxidant, dissolved in 70 mL of boiled deionized water, was added dropwise to the reaction system at a uniform rate within 2 hours, and the reaction was continued for 15 hours in a water bath at 80°C; the product was filtered with a glass sand funnel and then Wash and filter with 180mL deionized water for 3 times, and then vacuum dry at 120°C for 8 hours to obtain the product of the present invention. ICP and XRD analysis showed that the product composition was Li 0.99 MnO 2 , which is an orthorhombic layered lithium manganese oxide st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com