Diterpenoid monomer and its preparation method and application for preparing anti-cancer drugs
A technology of anticancer drugs and compounds, applied in the direction of drug combination, antineoplastic drugs, active ingredients of anhydride/acid/halide, etc., can solve the problems of unsatisfactory curative effect, low cancer curative effect, large toxic and side effects, etc., and achieve anticancer spectrum Wide range, high anti-cancer activity, less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the extraction method of ent-kauran-19-al-17-oic acid monomer ent-kauran-19-al-17-oic acid
[0022] ①The plant sample of custard apple was collected from Hainan and crushed after natural air-drying. The crushed custard apple stem was 3.5kg, extracted with 3000ml of 95% alcohol under reflux, recovered ethanol and then concentrated to obtain 300g of extract;
[0023] ② Extract the extract with chloroform to obtain a chloroform extract;
[0024] ③The chloroform extract was subjected to column chromatography through silica gel (100-200 mesh, 2kg), using 3000ml petroleum ether-chloroform (2:1) as eluent A to obtain the extract part;
[0025] ④The extract was subjected to column chromatography with 300g of silica gel (200-300 mesh), and eluted with 3000ml of petroleum ether-ethyl acetate (100:2) as eluent B, and each 50ml of eluent was a unit respectively Accepted, the eluent was subjected to silica gel thin-plate chromatography, the thin plate was sprayed with...
Embodiment 2
[0026] Example 2: Structural identification of ent-kauran-19-al-17-oic acid, a kaurane-type diterpene compound monomer:
[0027] ent-kauran-19-al-17-oic acid is a white powder, easily soluble in organic solvents such as chloroform and ethyl acetate, with a melting point of 199-203°C (CHCI 3 ). 1 HNMR suggested that ent-kauran-19-al-17-oic acid was a kaurane-type diterpene compound monomer.
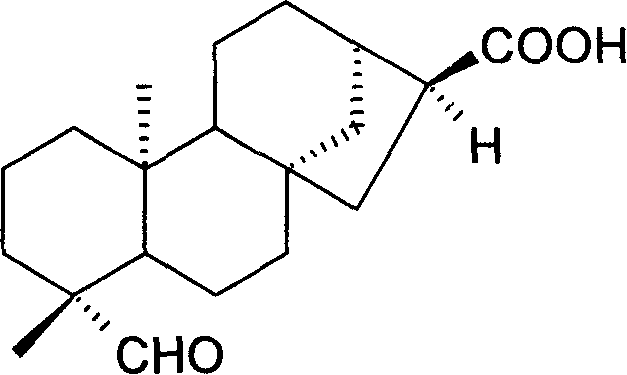
[0028] Molecular formula C 20 h 30 o 3 , its chemical structure is as follows:
[0029]
Embodiment 3
[0030] Example 3: Anti-liver cancer effect of diterpene compound monomer ent-kauran-19-al-17-oic acid
[0031] 1. Cell culture and compounds:
[0032] The human liver cancer cell line SMMC-7721 or HepG2 was routinely cultured in RPM11640 culture medium containing 10% inactivated calf serum, 100U / ml penicillin and 100ug / ml streptomycin at saturated humidity, 5% CO at 37°C 2 Incubator cultivation. The monomer compound is ent-kauran-19-al-17-oic acid, a smooth custard apple diterpene compound monomer identified by chemical structure.
[0033] 2. Experimental method:
[0034] Using conventional MTT method. Take SMMC-7721 or HepG2 liver cancer cells in the logarithmic growth phase, adjust the cell concentration to 1×10 with complete culture medium 5 / ml, inoculated cells in 96-well plates (1×10 4 After 24 hours, the experimental group was added with the diterpene compound ent-kauran-19-al-17-oic acid at a final concentration of 200ug / ml, 100ug / ml, 50ug / ml, 10ug / ml, and a 5-Fu g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com