Process for preparing alkannin or isoalkannin
A technique for shikonin and isoviolet, which is applied in the field of preparation of shikonin or isoshikonin, can solve the problems of low yield, many by-products, difficult post-processing and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
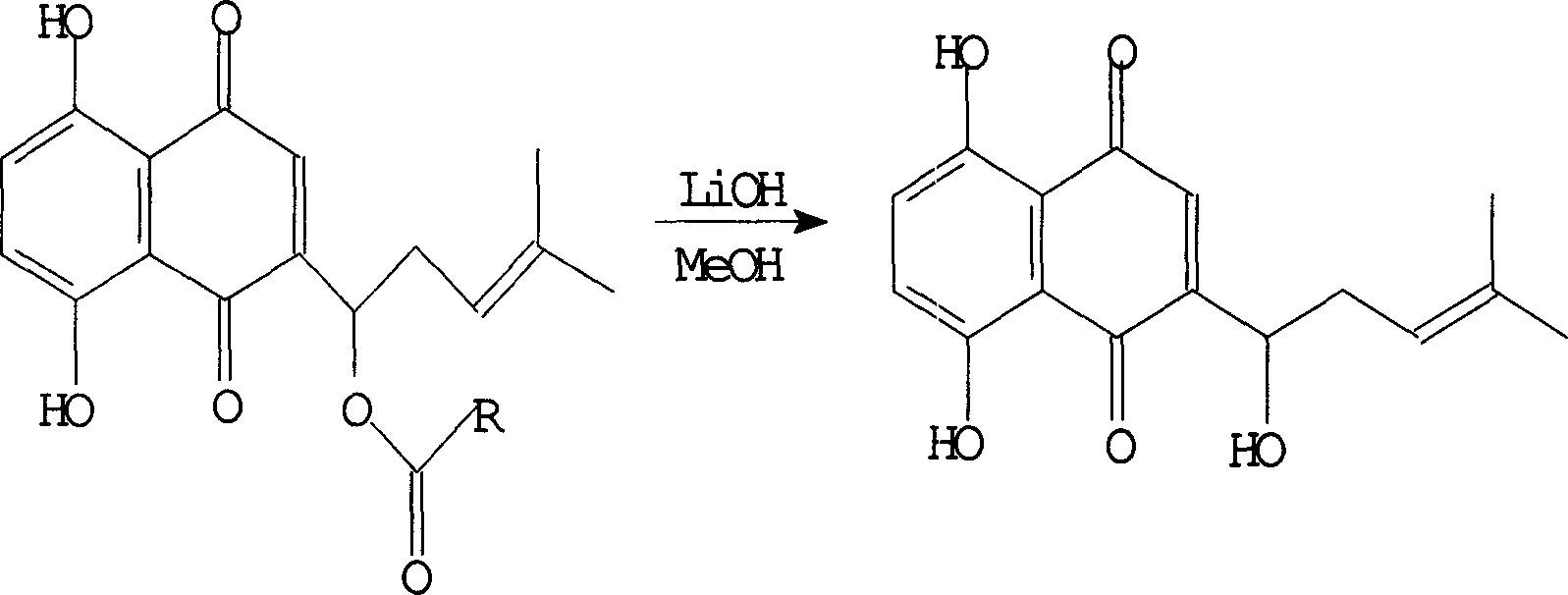
[0012] Weigh 400mg (1.08mmol) of β,β-dimethylacryloylshikonin, dissolve it in 5ml of methanol, pass it into nitrogen protection, cool the outside of the bottle with an ice-water bath to 0°C, add 387mg of lithium hydroxide (16.2mmol) After dissolving in methanol, add it dropwise to the flask, keep the temperature of the water bath at 0-8°C, and continue stirring for 72 hours. Sampling point plate, thin layer chromatography indicates that the reaction is over;
[0013] Add 10% aqueous hydrochloric acid solution dropwise to the reaction system, after the system turns completely red, add 10ml of water, extract with ethyl acetate (10ml×3), combine the extracts, and sequentially add aqueous sodium bicarbonate solution, water, and saturated sodium chloride The extract was washed with aqueous solution, dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure after drying to obtain a red shikonin solid, which was subjected to silica gel column chromato...
Embodiment 2
[0015] Weigh 4 g (10.81 mmol) of β, β-dimethylacryloyl isshikonin, dissolve it in 50 ml of methanol, pass through nitrogen protection, and cool the outside of the bottle with an ice-water bath to 0°C. Add 5.176g (21.62mmol) lithium hydroxide, keep the temperature of the water bath at 8-30°C, continue to stir for 120 hours, take a sample and spot the plate, and thin layer chromatography indicates that the reaction is complete;
[0016] Add 10% hydrochloric acid aqueous solution dropwise to the reaction system, after the system turns red completely, add 20ml of water, extract with ethyl acetate (30ml×3), combine the extracts, and successively add aqueous sodium bicarbonate solution, water, saturated sodium chloride The extract was washed with aqueous solution, dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure after drying to obtain a red isshikonin solid, which was subjected to silica gel column chromatography and eluted with petroleum eth...
Embodiment 3
[0018] Dissolve 1g of shikonin total quinones extracted from comfrey in 40ml of methanol, pass through nitrogen protection, cool the outside of the bottle with an ice-water bath to 0°C, dissolve 1.3g of lithium hydroxide in methanol, and add it dropwise to the flask. Keep the temperature of the water bath at 30-60°C, continue to stir for 72 hours, take a sample and point the plate, and thin-layer chromatography indicates that the ester compounds have reacted to form shikonin, and the non-ester compounds have no change;
[0019] Add 10% hydrochloric acid aqueous solution dropwise to the reaction system, after the system turns red completely, add 20ml of water, extract with ethyl acetate (30ml×3), combine the extracts, and successively add aqueous sodium bicarbonate solution, water, saturated sodium chloride The extract was washed with aqueous solution, dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure after drying to obtain a red shikonin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com