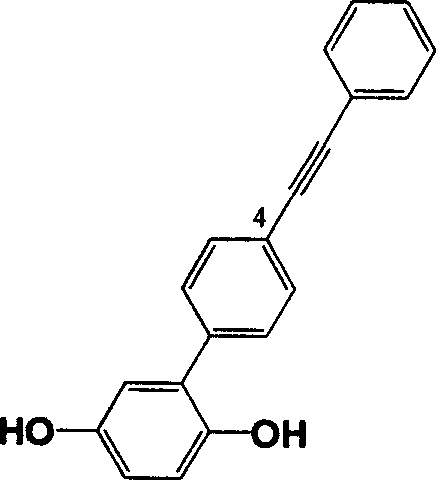
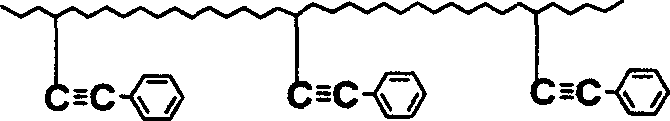
Preparation of monomer of dual functional groups containing lateral group phenylacetylene
A bifunctional group, phenylacetylene benzene technology, applied in the field of polymer materials and their synthesis, can solve the problem of damage to the mechanical properties of the polymer matrix
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of PEP-DF
[0047] 4,4'-difluorodiphenyl ketone (218g, 1mol), anhydrous K 2 CO 3 (28g, 0.2mol) and N, N-dimethylformamide (DMF) (1000ml), toluene (500ml) is put into the 3000ml that is equipped with nitrogen vent, constant pressure dropping funnel, belt water device, reflux condenser In a three-necked flask, resorcinol (11.0 g, 0.1 mol) dissolved in DMF was added dropwise to the reaction system under reflux of toluene, and water was added for 0.5 hours after the dropwise addition. Exclude toluene, then DMF reflux reaction for 4 hours, discharge into acidic water, filter the precipitate, wash with water, and dry. The unreacted 4,4'-difluorodiphenyl ketone was distilled off under reduced pressure, and then the residual component was extracted with ethanol to obtain a white trimer, which was dried in vacuum with a yield of 81%.
[0048] The above-mentioned trimer (50.6g, 0.1mol), p-bromobenzoyl chloride (26g, 0.12mol), and dichloromethane (...
Embodiment 2
[0051] PEP-DF (7.11g, 10mmol), resorcinol (1.1g, 10mmol), anhydrous potassium carbonate (2g) and N,N-dimethylformamide (25ml), benzene (25ml) were added to the container In a three-necked flask equipped with a nitrogen port, mechanical stirring, thermometer, water dispenser and reflux condenser, under the protection of nitrogen, heat to reflux with water for 2 hours. After excluding benzene, heat up to 150°C, react for 6 hours, pour the product into acidic water, pulverize and filter with a pulverizer, boil the solid directly with methanol and filter, repeat 5 times, then boil and filter with distilled water, repeat 5 times, in Dry in an oven to obtain a refined polyaryletherketone homopolymer with a yield of 92%.
Embodiment 3
[0053] Same as the method of embodiment 2, just change resorcinol into hydroquinone (or bisphenol A or other bisphenol monomers) or change DMF to be N, N-dimethylacetamide (or N- Methylpyrrolidone or sulfolane), benzene is changed to toluene, and the remaining methods are identical to obtain refined polyaryletherketone homopolymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com