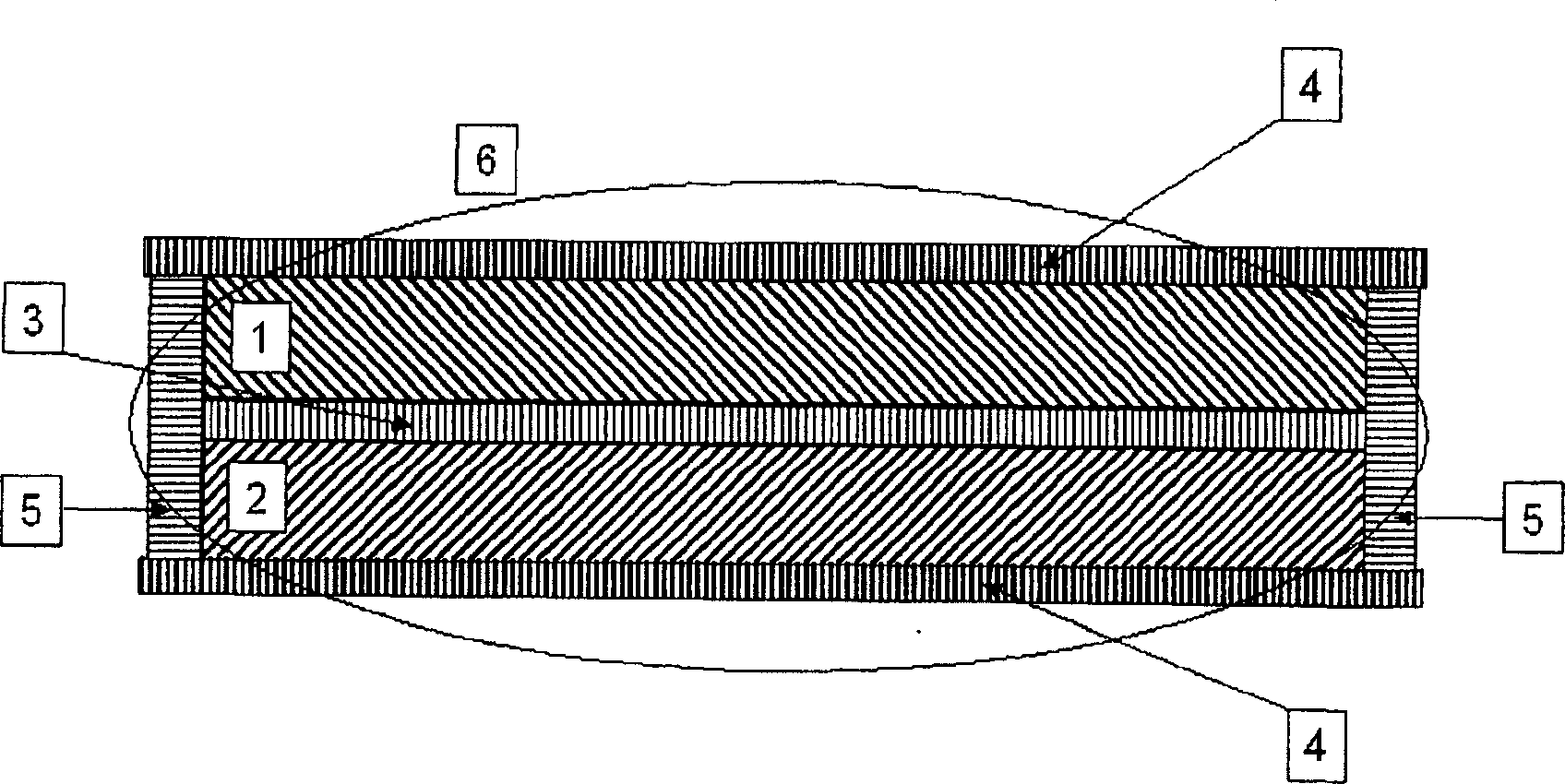
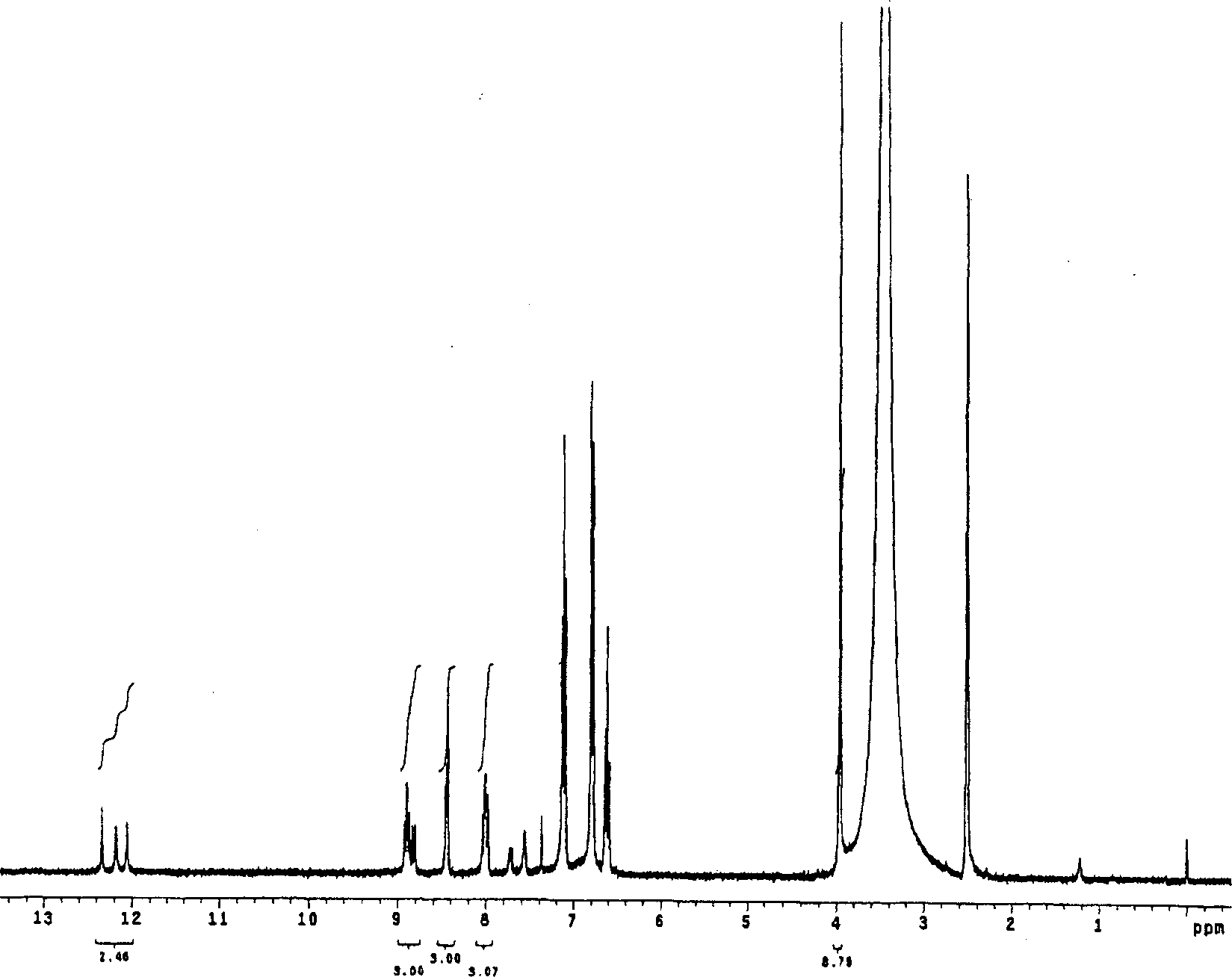
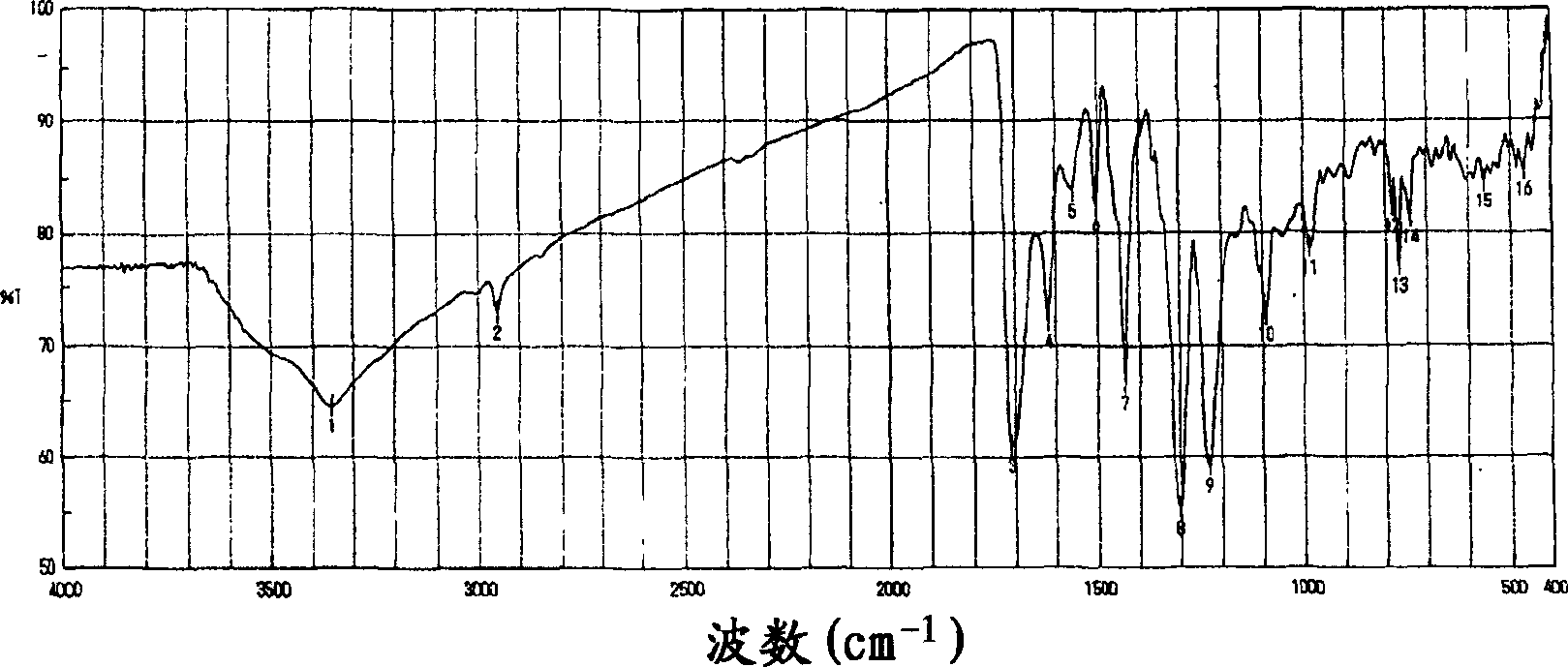
Indolecarboxylic ester trimer and electrochemical cell using the same
An indole carboxylate, trimer technology, used in organic chemistry, battery electrodes, optics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0115] Preparation Example 1 (synthesis of methyl 4-dimethylaminovinyl-3-nitrobenzoate)
[0116] Add 89.7 g of 4-methyl-3-nitrobenzoic acid methyl ester, 82.1 g of dimethylformamide dimethyl acetal and 200 ml of dimethylformamide (DMF) in the reaction flask, The contents were stirred at 120 °C for 6 hours. DMF was distilled off to obtain a reddish-purple solid product. Then, 300 ml of methanol was poured into the flask to wash the crystalline material and filtered. 104.8 g (91% yield) of methyl 4-dimethylaminovinyl-3-nitrobenzoate are obtained.
preparation Embodiment 2
[0117] Preparation Example 2 (synthesis of indole-6-carboxylic acid methyl ester monomer-catalytic reduction method)
[0118] 87.1 g of methyl 4-methyl-3-nitrobenzoate, 400 ml of methanol and 8 g of 5% palladium-carbon (Pd / C) were added to the reaction flask. The contents of the flask were stirred at room temperature under a hydrogen atmosphere for 12 hours at atmospheric pressure. The Pd / C was removed by filtration and the methanol was distilled off from the filtrate to give the product as a yellowish solid. The product was dissolved in ethyl acetate, washed with 5% aqueous NaOH, washed with 5% aqueous hydrochloric acid and water, and the ethyl acetate was distilled off. Thus, 50.4 g (68.7% yield) of methyl indole-6-carboxylate monomer was obtained.
preparation Embodiment 3
[0119] Preparation Example 3 (synthesis of indole-6-methyl carboxylate monomer-iron powder reduction method)
[0120] 176.4 g of iron powder, 43.5 g of acetic acid, 71.2 g of water and 273.3 g of toluene were added to the reaction flask. The contents of the flask were heated to 80°C. Then, a solution of 131.8 g of methyl 4-methyl-3-nitrobenzoate in 110.4 g of DMF was added dropwise to the above-mentioned mixed solution during about 1 hour, and then the resulting mixture was stirred again at 80 degrees Celsius for 4 Hour. After cooling the reaction mixture, solid material was removed by filtration. The filtrate was washed with 5% aqueous NaOH, then 5% aqueous hydrochloric acid and water, after which the toluene was distilled off to give the product as a brown solid. Then, 164.3 g of cyclohexane was added to dissolve the solid product at about 80 degrees Celsius. The resulting solution was cooled to room temperature, and the precipitated crystalline substance was collected b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com