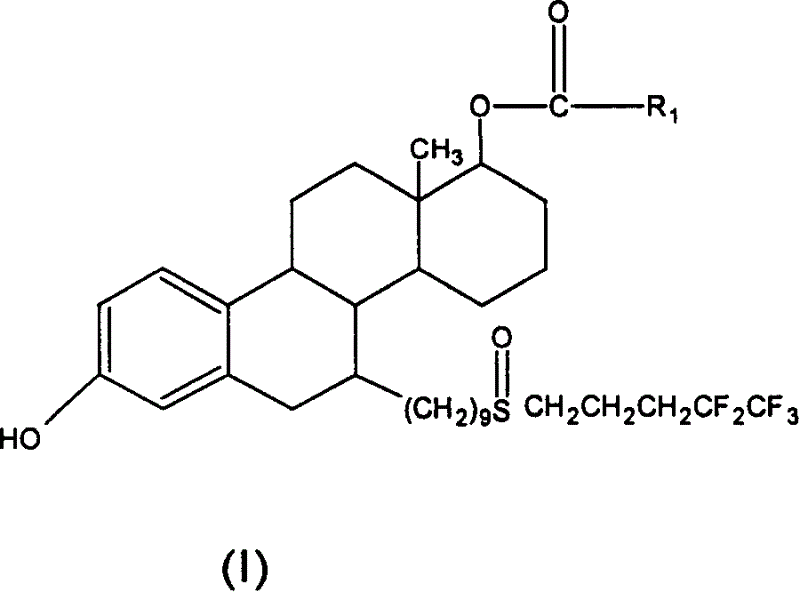
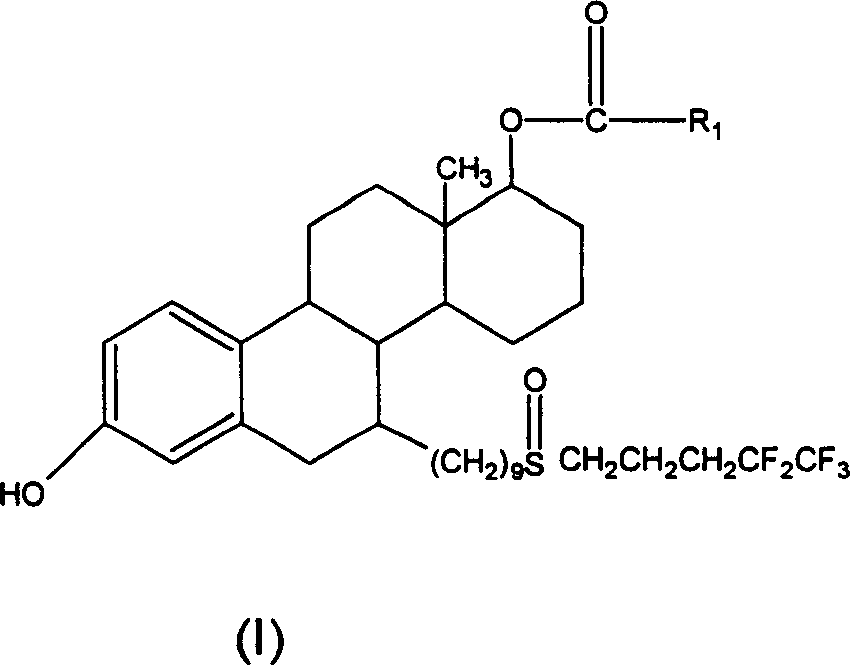
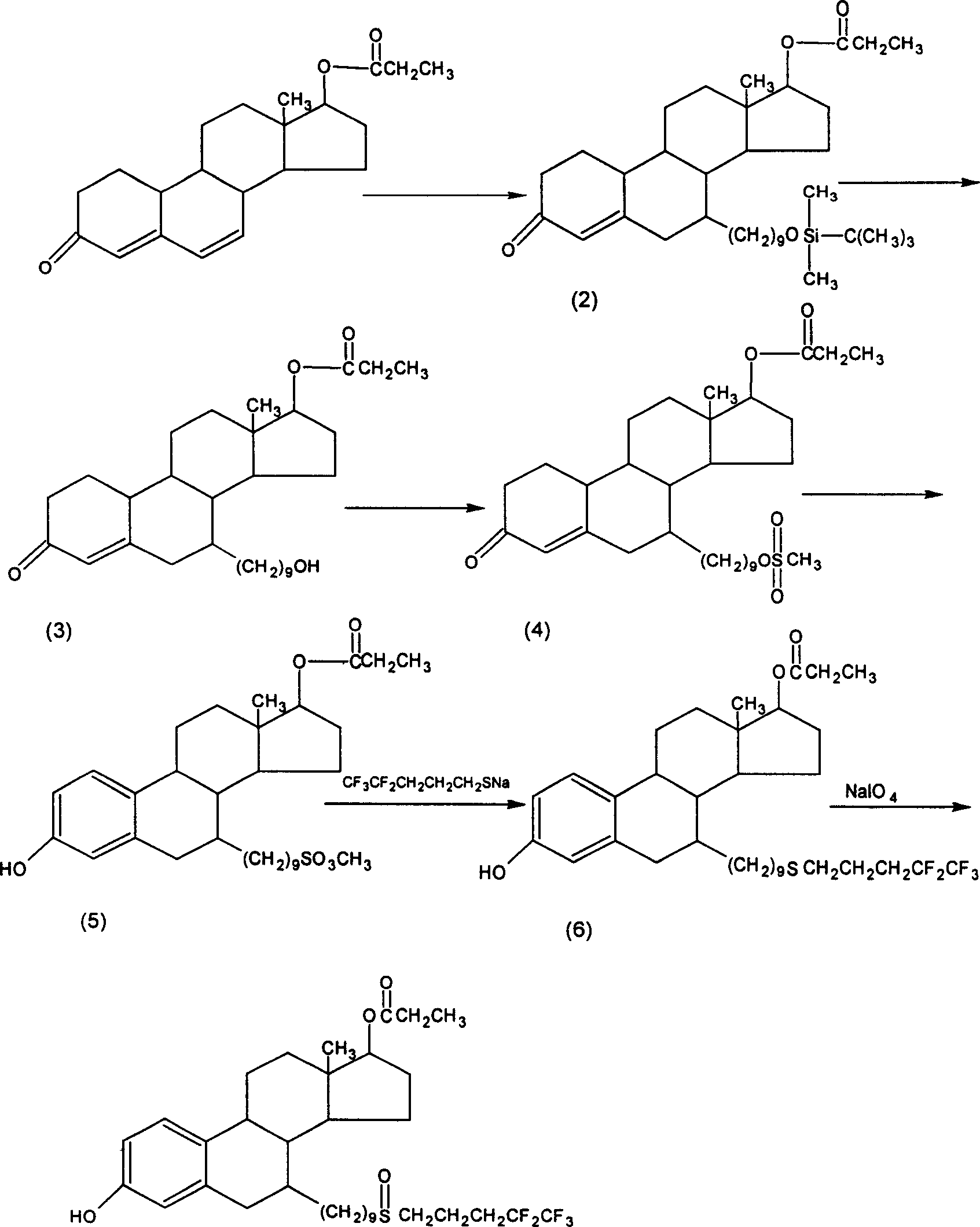
Steroid derivatives
A technology of drugs and compounds, applied in the field of steroid derivatives, to achieve the effect of reducing side effects and blocking stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of 17-β-propionyloxy-7α-[9-(dimethyl-tert-butylsiloxy)nonyl]-estr-4-en-3-one
[0036]In a 500 ml reaction flask, drop magnesium chips (2.84 grams) and THF (40 milliliters), add dropwise a mixed solution of bromosilyl ether (41 grams) and THF (140 milliliters) under stirring, and react at room temperature for 3 About an hour until the action of magnesium shavings is complete. Cool to internal temperature -30°C, add cuprous chloride (1 g), stir for 5 minutes, then add dropwise the mixed solution of intermediate (II) (9.2 g) and THF (50 ml), after dropping, keep warm - React at 35°C for 1 hour. After the reaction, add 100 ml of glacial acetic acid to the reaction solution. After stirring completely, add diethyl ether (300 ml) and water (200 ml), separate layers, and reverse the aqueous layer with diethyl ether (100 ml*2). Extracted, combined organic layers, washed with water (100 ml), brine (100 ml*2) successively, dried, filtered and concentrated to dryne...
Embodiment 2
[0048] The compound of the present invention and fulvestrant have been done drug effect contrast research, by experiment, have drawn following data and conclusion:
[0049] group
dosage
(mg / piece)
way
number of animals
d0 dn
weight
(gram)
d0 dn
TV
x±SD
d0 dn
RTV
x±SD
T / C
(%)
NS
S.C.
6 4
20 20
66±11 133±34
2.1±0.5
Example 1
5
S.C.
53
22 22
62±11 98±20
1.7±0.5
79.3
Example 1
3
S.C.
64
20 20
39±21 76±9
1.9±0.7
90.0
[0050] d0: The time of drug administration in separate cages
[0051] dn: the time when the actual therapeutic effect is the best, this experiment is 28 days after the first administration
[0052] TV: tumor volume
[0053] RTV: relative tumor volume (V n / V 0 )
[0054] T / C: Anti-tumor activity e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com