Oleanolic acid and its derivative, preparation method and use
A technology of oleanolic acid and derivatives is applied in the fields of oleanolic acid and derivatives thereof, preparation method and use, and can solve the risk of breast cancer and endometrial cancer, and the long-term use effect of bisphosphonates is not completely confirmation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
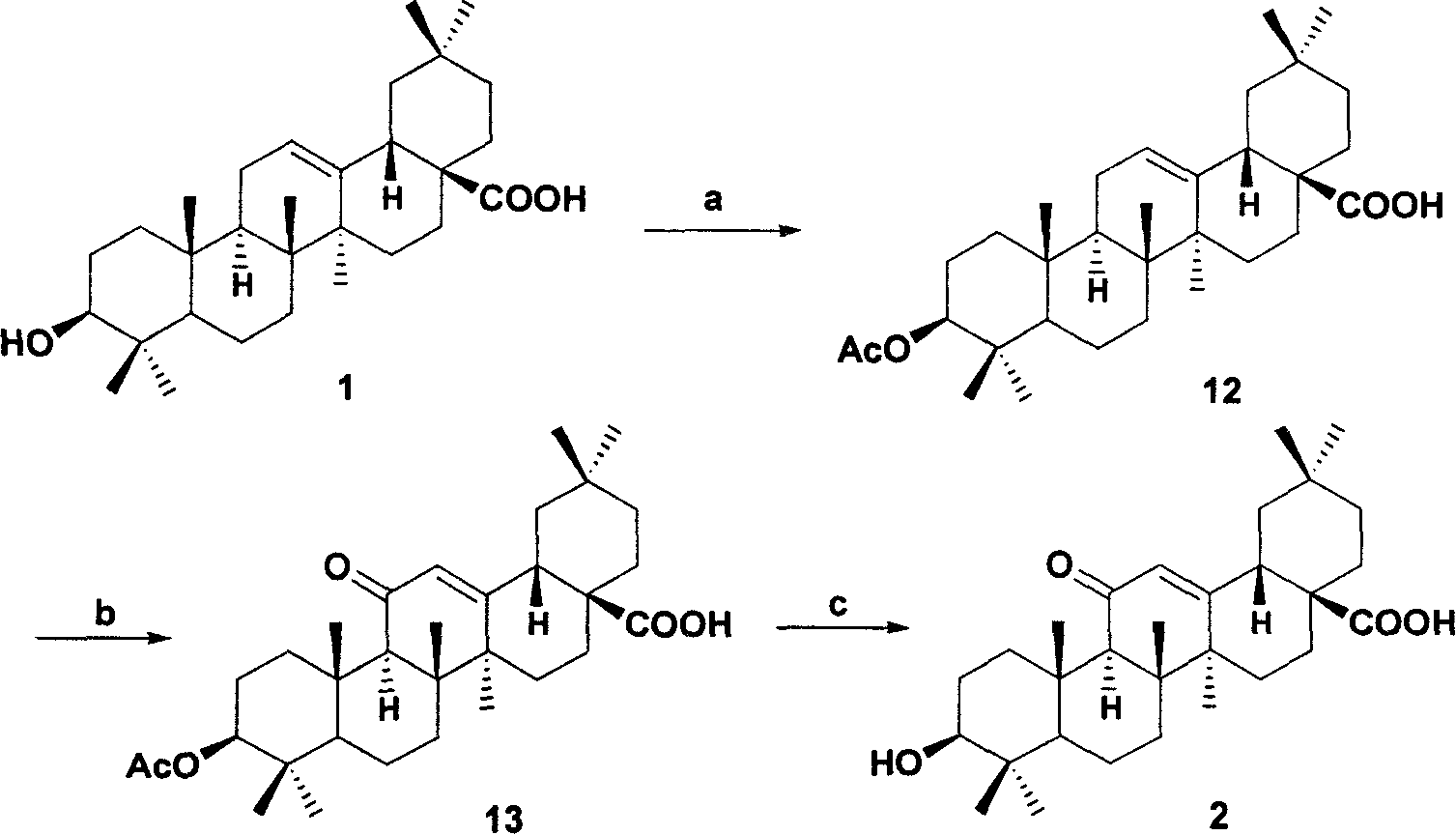
[0035] Example 1: The 3-hydroxyl of oleanolic acid 1 is protected with acetyl, and then the methylene at the 11-position is oxidized with the reagent of (b), and the protective group is removed to obtain derivative 2, as follows:
[0036] a: Add 4.57g (10mmol) oleanolic acid 1 to 19mL pyridine, add 9.45mL acetic anhydride dropwise under ice bath stirring, remove the ice bath after dissolving, add 122mg (1mmol) DMAP, react for about 2h, TLC (acetic acid ethyl ester: petroleum ether=1: 3, V: V) to detect the reaction progress, after the reaction, the reaction solution was poured into 50mL of ice water, and washed with 20mLCH 2 Cl 2 Extract three times. The extract was washed with 5% HCl, water, and saturated brine respectively, the organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated to obtain 4.93 g of 3-O-acetyl oleanolic acid 12 with a yield of 99%.
[0037] b: 45mLCCl 4 5.8g of 12 was added in , and 11.2mL of glacial acetic acid ...
Embodiment 2
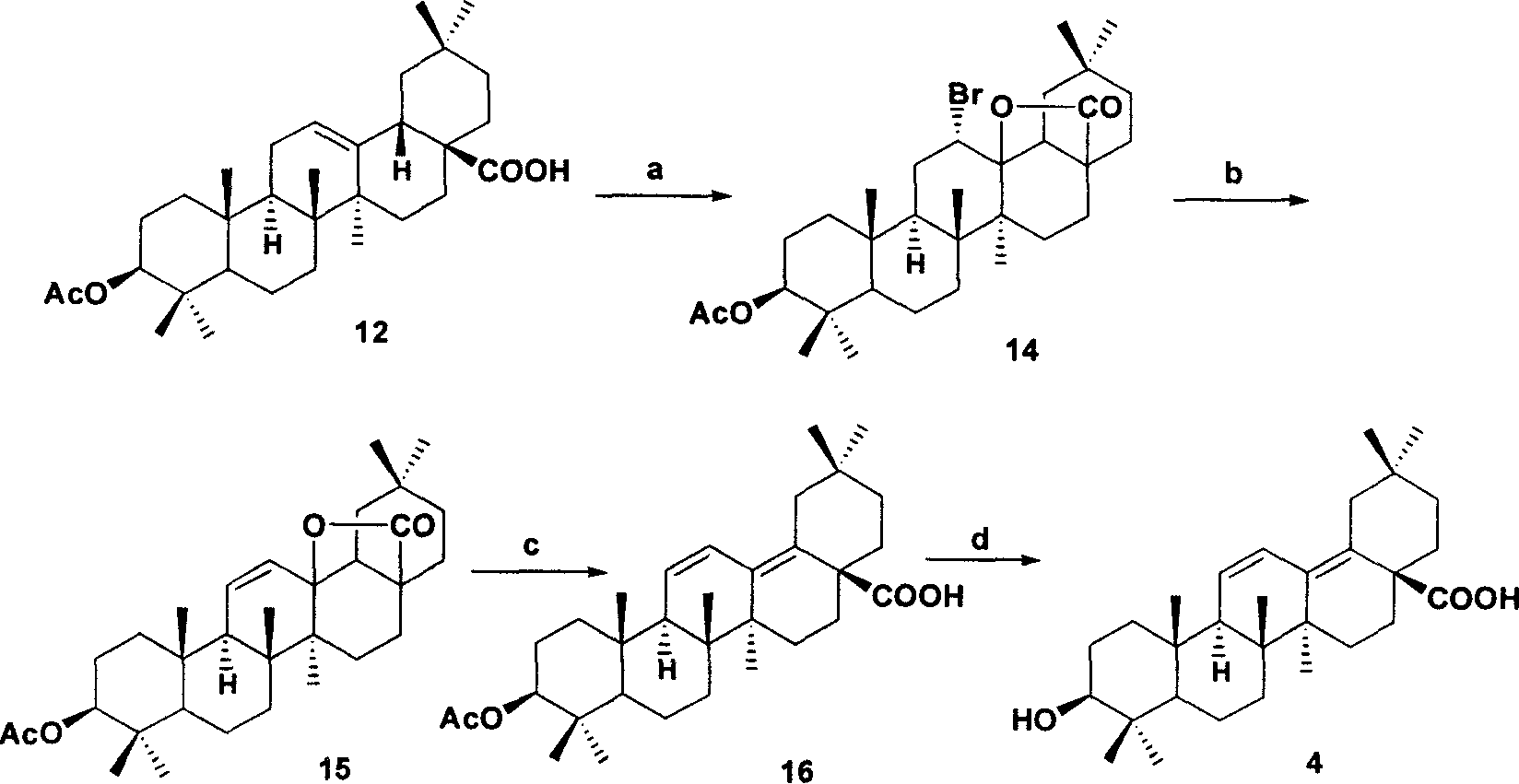
[0040] Example 2: 13Δ 11-12 ,Δ 14-18 Preparation of oleanolic acid 4:
[0041] a. Add 0.996g (2mmol) 12 to 50mL methanol, add 1.25gBr dropwise 2 (7.7mmol) dissolved in 50mL methanol solution. After stirring at room temperature for 0.5 h, the mixture was cooled in an ice bath and filtered to obtain 0.7 g of 14 with a yield of 63%.
[0042] b: Add 6g of 14 to 100mL of o-xylene, dissolve it, add 23mL of DBU, and stir under reflux for 16h. The reaction solution was separated with ether and water, and the organic layer was washed with 5% HCl, saturated NaHCO3 and saturated brine, and dried over anhydrous sodium sulfate. After filtering, part of the solvent was evaporated, cooled and left standing, crystals were precipitated, and filtered to obtain 3.84g of 15 with a yield of 72%.
[0043] c: Add 3 g of 15 to 100 mL of 9% HCl in methanol and stir at room temperature for half an hour. TLC (CHCl3:petroleum ether=1:1) detected the progress of the reaction. After the reaction was...
Embodiment 3
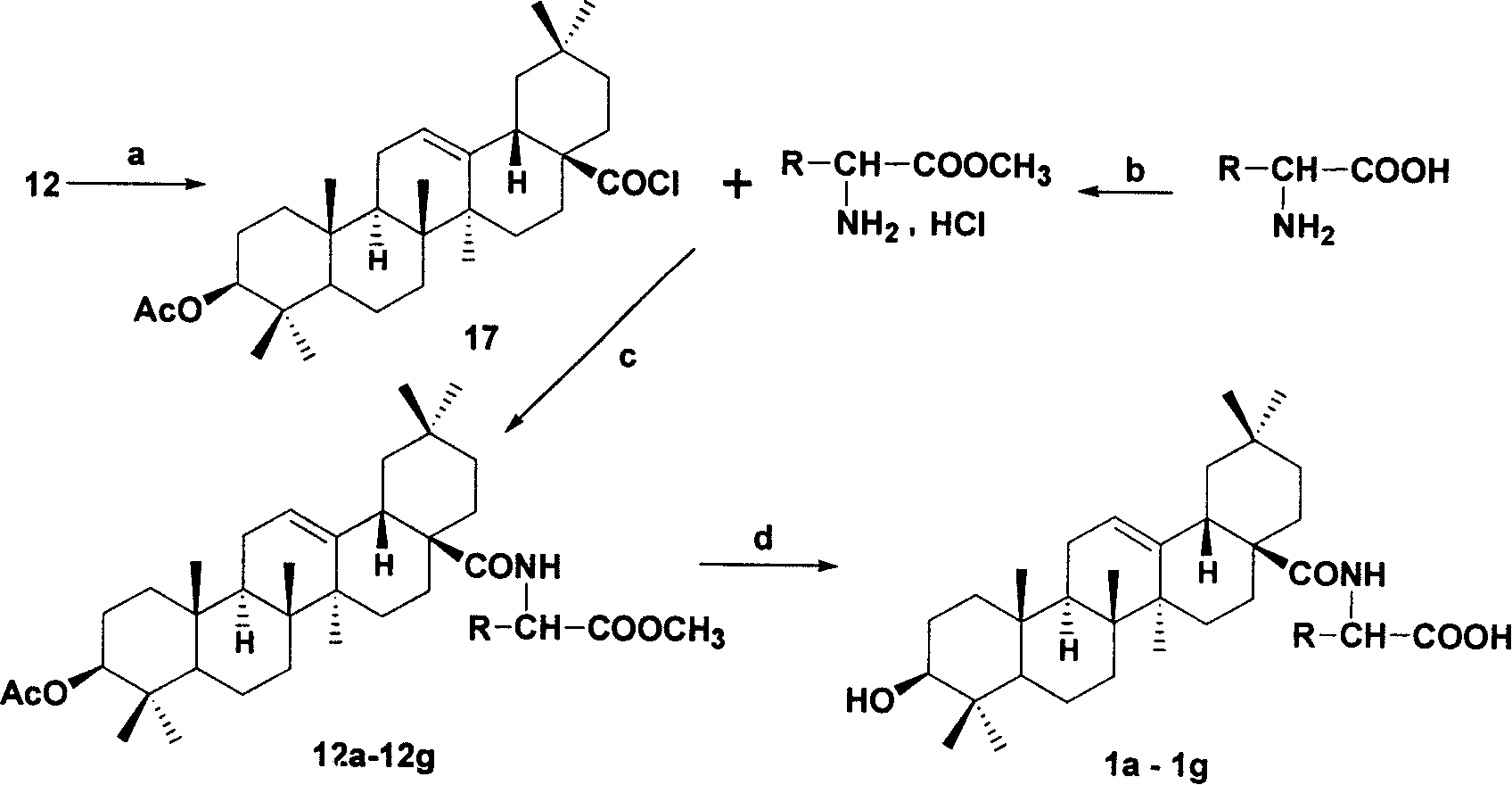
[0045] Example 3: Acylate the carboxyl group of oleanolic acid derivative 12 using the conditions in the figure (a) below to obtain compound 17, and at the same time prepare the amino acid methyl ester hydrochloride protected by the amino acid carboxyl group with the reagent (b) . Reaction of 17 and amino acid methyl ester hydrochloride under the conditions of (c) to obtain derivatives 12a-12g, deprotection under the conditions of the following figure (d) to obtain derivatives 1a-1g. The amino acids used therein are all naturally occurring amino acids and artificially synthesized D-type amino acids.
[0046] a: Dissolve 6.00 g (12 mmol) of 12 in 80 mL of dichloromethane, then add 4.4 mL of oxalyl chloride dropwise, and react at room temperature for 24 h. After the reaction, the solvent was evaporated to dryness, then 5×300 mL cyclohexane was added, and evaporated to dryness to obtain 3-O-acetyl oleanene-28-acyl chloride 17. Proceed directly to the next reaction.
[0047] c:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com