Compound of butenafine hydrochloride, preparation method, and application as medication for restraining and killing fungus
A technology of butenafine hydrochloride and its compound, which is applied in the field of compound and its preparation, can solve the problems of single mechanism of action, decreased therapeutic effect, high recurrence rate of symptoms, etc., and achieve long action time, low drug resistance and low recurrence rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
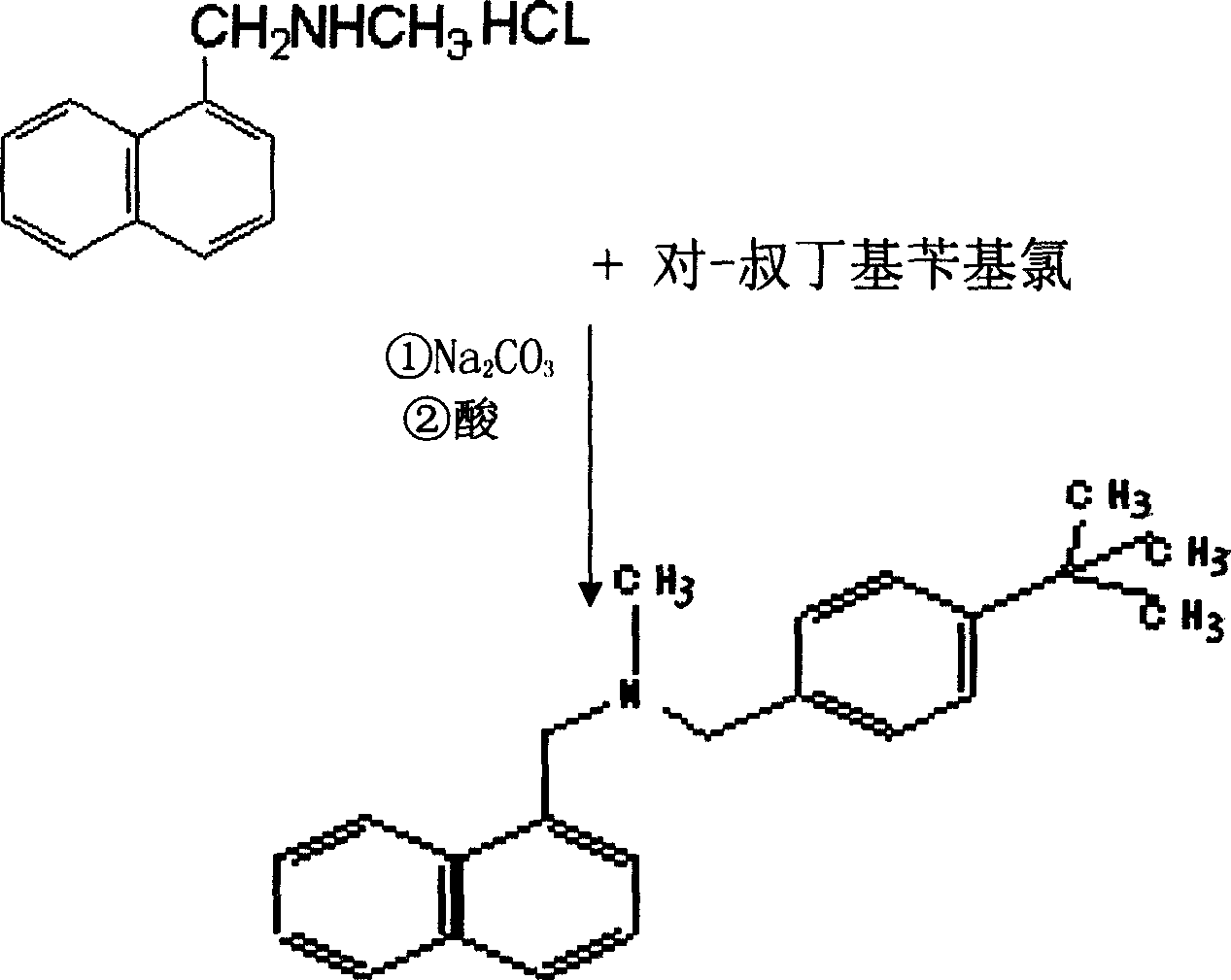
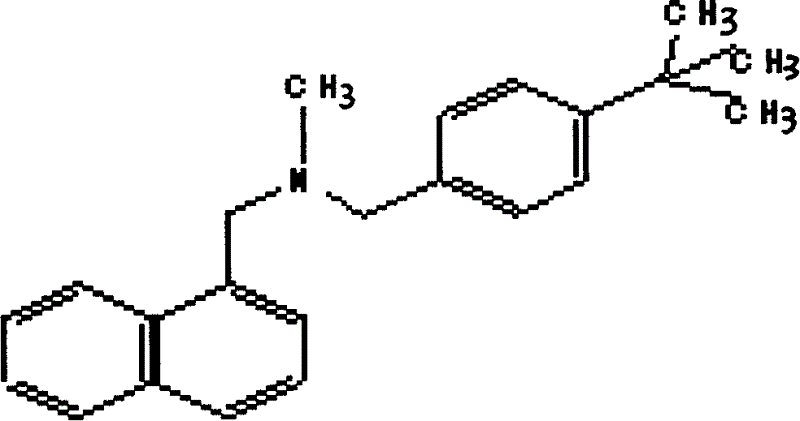
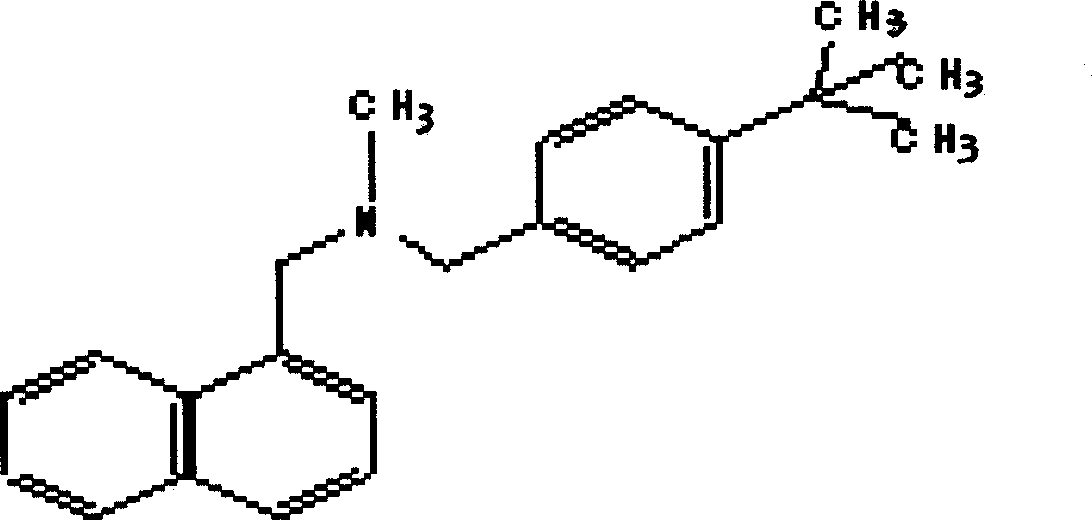
[0027] Such as figure 1 As shown, N-methyl-1-naphthylmethylamine, p-tert-butylbenzyl chloride, anhydrous sodium carbonate, DMF (dimethylformamide) were purchased from the market, and 685g (4.0mol) N- Methyl-1-naphthylmethylamine, 730g (4.0mol) of p-tert-butylbenzyl chloride, 424g (4.0mol) of anhydrous sodium carbonate and 3000ml of DMF were successively put into the reaction flask, and stirred and reacted at 50-60°C for 16 hours. After cooling, pour the reaction mixture into an appropriate amount of ice water; add acid to make it strongly acidic, and precipitate a solid, cool, filter the solid, and recrystallize with ethanol-ethyl acetate to obtain 1100 g of a white solid product, which is the target compound cloth hydrochloride. For tenafine, the yield is 77.7%. The added acid can be selected from sulfuric acid, nitric acid or hydrochloric acid, and concentrated sulfuric acid is selected in this embodiment.
[0028] Prepare liniment according to the following prescription w...
Embodiment 2
[0034] 343g (2.0mol) of N-methyl-1-naphthylmethylamine, 365g (2.0mol) of p-tert-butylbenzyl chloride, 212g (2.0mol) of anhydrous sodium carbonate and 1500mlDMF were dropped into the reaction flask successively, at 50 The reaction was stirred at -60°C for 16 hours. After cooling, the reaction mixture was poured into ice water; nitric acid or hydrochloric acid was added to make it strongly acidic, and a solid was precipitated. Cool, collect the solid by filtration, and recrystallize with ethanol-ethyl acetate to obtain 550 g of a white solid product which is the target compound butenafine hydrochloride, with a yield of 77.7%.
[0035] Buy stearic acid, white petrolatum, glyceryl monostearate, polysorbate, propylene glycol, methyl cellulose, ethylparaben from the market, and use the prepared butenafine hydrochloride to prepare milk according to the following prescription Ointment (percentage by weight): 0.08-0.1% butenafine hydrochloride, 9-12% stearic acid, 7-10% white petrolat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com