Fluorouracil-dextran and process for preparing the same
A technology of fluorouracil and dextran, which is applied in the field of anti-tumor macromolecule prodrug-fluorouracil-dextran conjugate and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
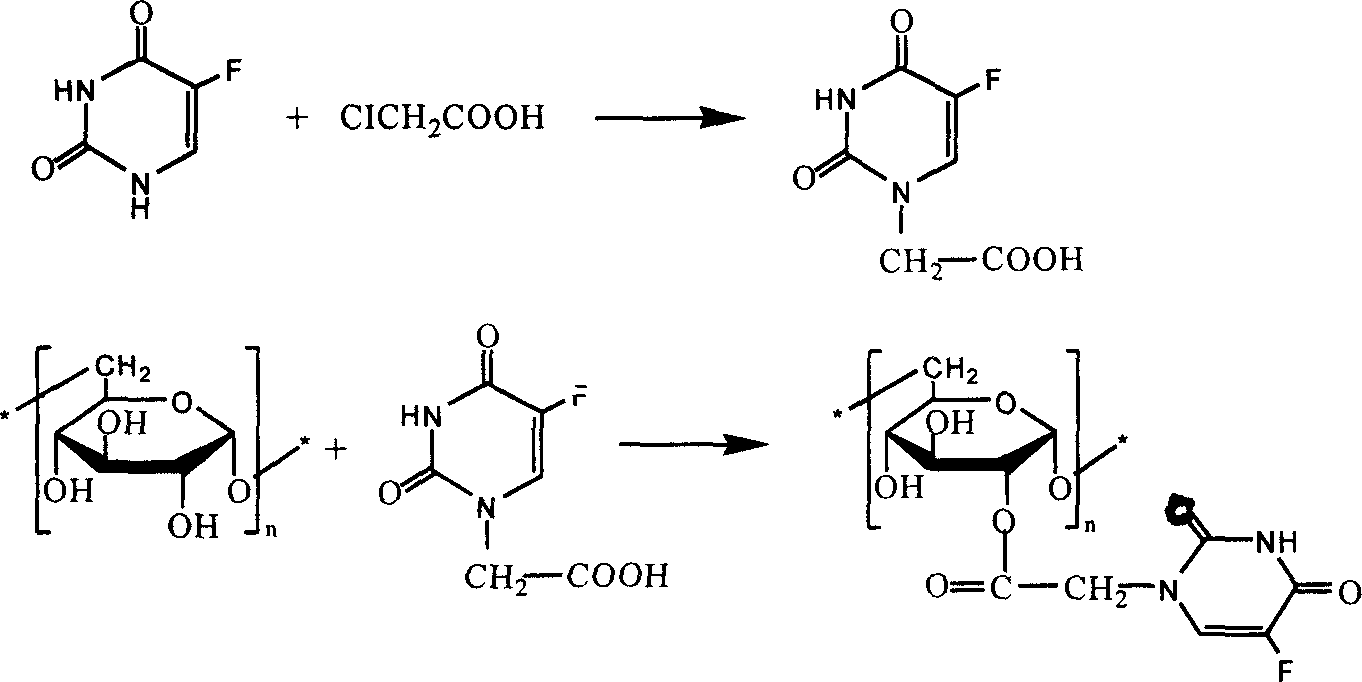
[0026] 1. Synthesis of fluorouracil-1-acetic acid
[0027] Dissolve 13.0g of fluorouracil in 75ml of water containing 11.2g of potassium hydroxide, add 40ml of aqueous solution containing 9.5g of chloroacetic acid, adjust the pH value to greater than 10 with potassium hydroxide, heat up and reflux for 2 hours, cool to room temperature, and acidify with concentrated hydrochloric acid , a precipitate was obtained, filtered, and recrystallized with water to obtain 8.0 g, yield 50%.
[0028] 2. Synthesis of fluorouracil-dextran
[0029] 1g (6.17mmol of anhydrous glucose unit) dextran was dissolved in 20ml of mixed solvent (formamide: dimethylformamide: dichloromethane = 10:9:1 (v / v / v)), kept at 30°C, added fluorouracil - 1.16g (6.17mmol) of 1-acetic acid, after dissolving, add 75mg DMAP, stir to dissolve, add 1.27g (6.17mmol) of dicyclohexylcarbodiimide, and keep warm at 30°C for 24 hours. Remove the generated dicyclohexylurea by filtration, pour the filtrate into 160ml of metha...
Embodiment 2
[0031] Synthesis of Fluorouracil-Dextran Conjugates
[0032]Dissolve 1g of carbonyldiimidazole in tetrahydrofuran, add 1.16g of fluorouracil-1-acetic acid at room temperature, stir for 4 hours, evaporate the solvent under reduced pressure, dissolve the solid in 20ml of DMSO, add an appropriate amount of triethylamine, and then add 1g (6.17mmol Water glucose unit) dextran is dissolved in the solution of DMSO of 20ml, keep warm at 30 ℃, react for 24 hours. The reaction solution was poured into 160ml of methanol:ether (3:1, v / v) mixed solvent to precipitate, collected by filtration, washed successively with 50ml of ether and 50ml of acetone, dried at 30°C in the presence of phosphorus pentoxide, and kept dry stored in the device.
Embodiment 3
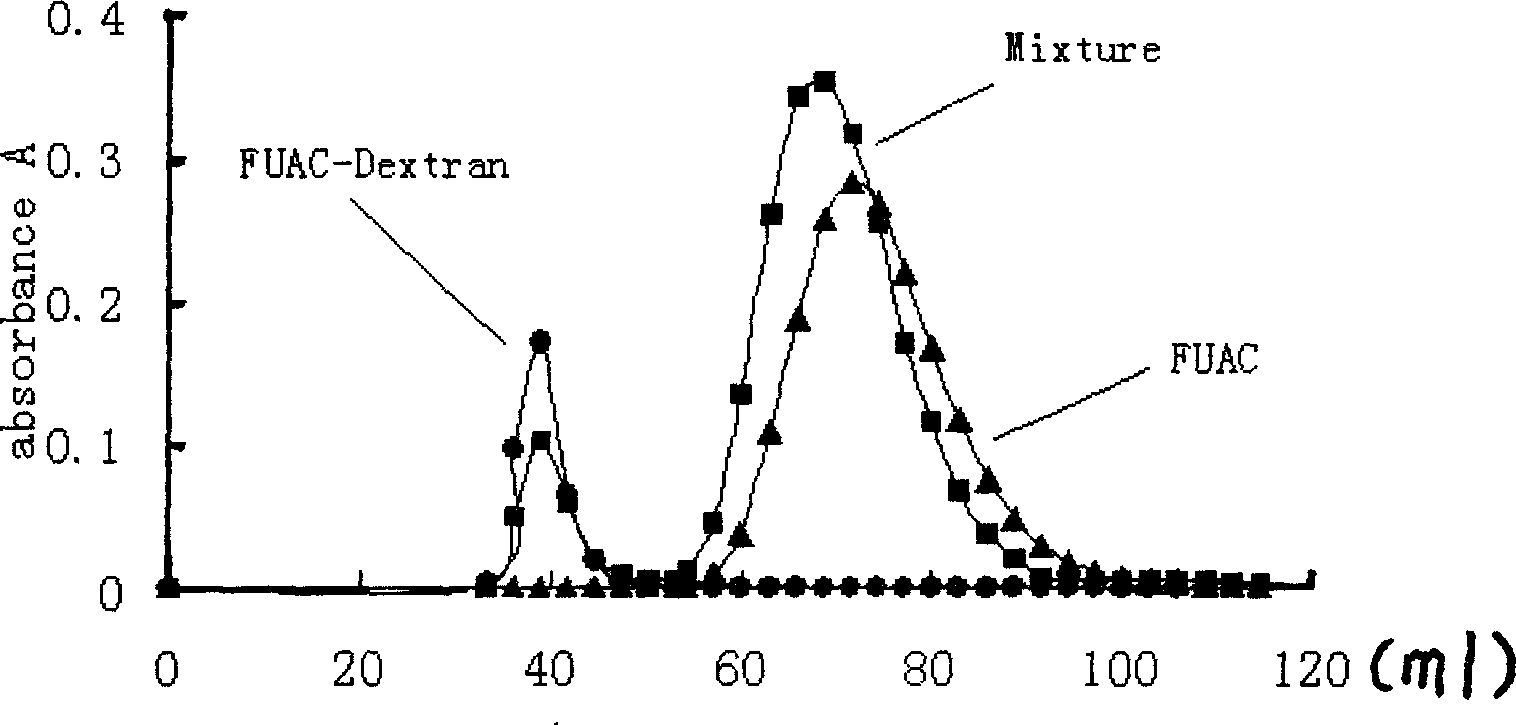
[0034] In vitro stability of fluorouracil-dextran conjugates
[0035] Prepare hydrochloric acid, citrate, acetate, phosphate and borate buffers with a concentration of 0.1M and pH values of 1.24, 2.96, 4.95, 6.80, and 8.75, and add an appropriate amount of NaCl to adjust the total ions of each buffer The strength is 0.5M. Take 9.5ml of each of the above solutions, put them on a constant temperature water bath at 60°C, preheat for 30min, add 0.5ml of FT-70 aqueous solution of appropriate concentration (FUAC substitution degree is 15.1%), so that the final FUAC (calculated by FUAC) concentration is 50μM, respectively 200 μl samples were taken at different time points, and the contents of FUAC and Fu were analyzed by high performance liquid chromatography. The hydrolysis constants of the conjugates in different buffers were calculated from the contents of FUAC and Fu in the reaction solution at different times.
[0036] It can be seen from the test results that the precursor ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com