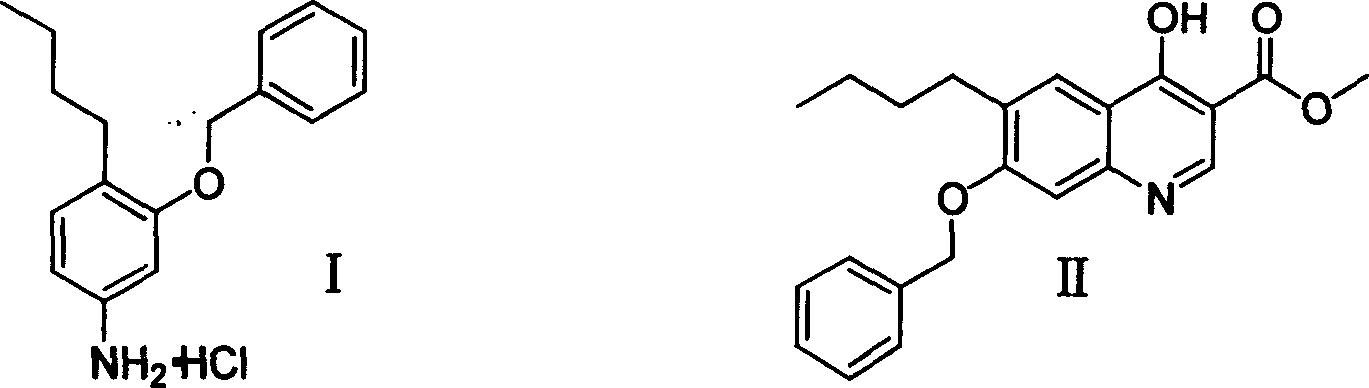
3-benzyloxy-4-butylaniline hydrochloride preparation method
A technology of butylaniline and butyrylaniline, which is applied in the field of preparation of 3-benzyloxy-4-butylaniline hydrochloride, can solve the problems of difficult separation and purification, difficult complete reaction and high reaction temperature, and achieves easy Purification, good product purity, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Preparation of 3-butyrylaminophenol butyrate
[0027] Add 50g (0.46mol) of m-aminophenol, 128ml (0.92mol) of triethylamine, and 200ml of dichloroethane to a 500ml four-neck flask in turn, add 100ml (0.98mol) of butyryl chloride dropwise at room temperature, add it in 30 minutes, and reflux The reaction was carried out for 4 hours. Stop the reaction, cool slightly, filter with suction, and rinse the filter cake with 100ml of dichloroethane; then pour the filtrate into a 1L separatory funnel, add 400ml of water, wash with water for 3 times, dry the organic layer with anhydrous sodium sulfate, and concentrate the organic layer , recovery of dichloroethane to obtain oily liquid 110g, yield 96.4%.
[0028] 2) Preparation of 2-butyryl-5-butyrylaminophenol
[0029] In a 500ml four-necked flask, add 94g (0.38mol) 3-butyrylaminophenol butyrate, 101g (0.76mol) AlCl 3 And dichlorobenzene (200ml), heated up to 140 degrees to react for 4 hours, cooled, slowly poured into 200ml...
Embodiment 2
[0037] 1) Preparation of 3-butyrylaminophenol butyrate
[0038] Add 50g (0.46mol) of m-aminophenol, 36.8g (0.92mol) of sodium hydroxide, and 200ml of ethyl acetate in sequence in a 500ml four-neck flask, add 154.8g (0.98mol) of butyrylic anhydride dropwise at room temperature, and complete the addition in 30 minutes , reacted under reflux for 4 hours. Stop the reaction, cool slightly, filter with suction, and rinse the filter cake with 100ml of dichloroethane; then pour the filtrate into a 1L separatory funnel, add 400ml of water, wash with water for 3 times, dry the organic layer with anhydrous sodium sulfate, and concentrate the organic layer , and recover ethyl acetate to obtain 106 g of oily liquid with a yield of 92.9%.
[0039] 2) Preparation of 2-butyryl-5-butyrylaminophenol
[0040] In a 500ml four-necked flask, add 94g (0.38mol) 3-butyrylaminophenol butyrate, 101g (0.76mol) AlCl 3 and sodium chloride (20g), heated to 120°C for 4 hours, cooled to 90°C, slowly added 20...
Embodiment 3
[0048] 1) Preparation of 3-butyrylaminophenol butyrate
[0049] Add 50g (0.46mol) of m-aminophenol, 127g (0.92mol) of potassium carbonate, and 200ml of toluene into a 500ml four-neck flask in sequence, and add 100ml (0.98mol) of butyryl chloride dropwise at room temperature, complete the addition in 30 minutes, and react under reflux for 5 hours . Stop the reaction, cool down, filter with suction, and rinse the filter cake with 100ml of toluene; then pour the filtrate into a 1L separatory funnel, add 400ml of water, wash with water for 3 times, dry the organic layer with anhydrous sodium sulfate, concentrate the organic layer, and recover the toluene , to obtain 98 g of oily liquid, yield 85.9%.
[0050] 2) Preparation of 2-butyryl-5-butyrylaminophenol
[0051] In a 500ml four-necked flask, add 94g (0.38mol) 3-butyrylaminophenol butyrate, 101g (0.76mol) AlCl 3 and 1,2-dichloroethane (200ml), warming up to 75°C for 6 hours, cooling, slowly adding 200ml of hydrochloric acid a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com