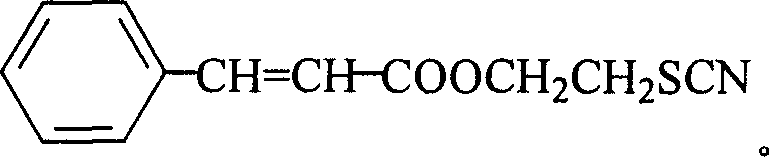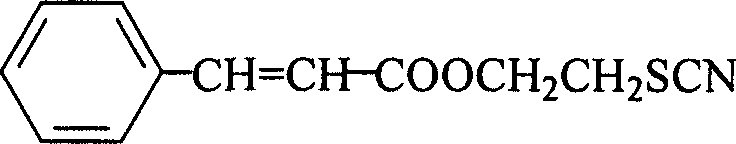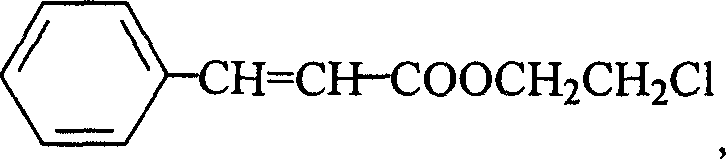(2'-thiocyano) ethyl cinnamate compound and its preparing method and use
A technology of phenylacrylic acid and compound, which is applied in the field of cinnamic acid derivative compound and its preparation, can solve the problems of not very high antibacterial performance and poor microbial inhibition effect, achieve a wide range of use, less waste discharge, and improve antibacterial and mildew resistance performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Take 60g (0.4mol) of 3-phenylacrylic acid, 135ml (2.0mol) of 2-chloroethanol and 4.8g (0.04mol) of sodium bisulfate into a 500ml round bottom flask, stir, heat to boiling, and reflux at constant temperature After 4h, distill and reclaim the unreacted 2-chloroethanol; then, in the remaining reactant, add a concentration of 10% sodium carbonate aqueous solution to make the pH value of the reactant 6.0, then extract the reactant with 200ml ether, and Wash the ether layer with water to make the pH value 6.0; then add 50 g of anhydrous sodium carbonate to the ether layer, remove water, let stand for 3 hours, and filter to remove sodium carbonate; transfer the ether layer to a distillation apparatus, distill and Ethyl ether was recovered, and the residual substance was distilled under reduced pressure at a pressure of 0.095 MPa and a temperature of 120° C. to obtain 72.0 g of (2′-chloro)ethyl 3-phenylacrylate with a yield of 85.5%.
[0041]2. Take 30g (0.14mol) of (2′-chlo...
Embodiment 2
[0043] 1. Take 60g (0.4mol) of 3-phenylacrylic acid, 243ml (3.6mol) of 2-chloroethanol and 2.4g (0.02mol) of sodium bisulfate into a 500ml round bottom flask, stir, heat to boiling, and reflux at constant temperature After 8h, distill and reclaim the unreacted 2-chloroethanol; then, add a concentration of 10% sodium bicarbonate aqueous solution to the remaining reactant to make the pH value of the reactant 8.0, then extract the reaction with 300ml dichloromethane and wash the dichloromethane layer with water to make the pH value 6.5; then add 50 g of anhydrous magnesium sulfate to the dichloromethane layer, remove water, let stand for 3 hours, and filter to remove magnesium sulfate; dichloromethane The layer is transferred to the distillation unit, distilled and reclaimed dichloromethane, the residual material is at a pressure of 0.095MPa, and at a temperature of 120°C, after distillation under reduced pressure, 70.0g of the (2'-chloro)ethyl 3-phenylacrylate product is obtained...
Embodiment 3
[0046] 1. Take 60g (0.4mol) of 3-phenylacrylic acid, 189ml (2.8mol) of 2-chloroethanol and 3.84g (0.032mol) of sodium bisulfate into a 500ml round bottom flask, stir, heat to boiling, and reflux at constant temperature After 6h, distill and reclaim the unreacted 2-chloroethanol; then, add concentration to the remaining reactant and be 10% potassium carbonate aqueous solution, make the pH value of reactant be 7.5, then extract reactant with 250ml chloroform, and Wash the chloroform layer with water to make the pH value 7.0; then add 50 g of anhydrous sodium sulfate to the chloroform layer, remove water, let stand for 3 hours, and remove sodium sulfate by filtration; transfer the chloroform layer to a distillation apparatus, distill and Chloroform was recovered, and the residual substance was distilled under reduced pressure at a pressure of 0.095 MPa and a temperature of 120° C. to obtain 73.6 g of (2′-chloro)ethyl 3-phenylacrylate with a yield of 87.4%.
[0047] 2. Take 30g (0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com